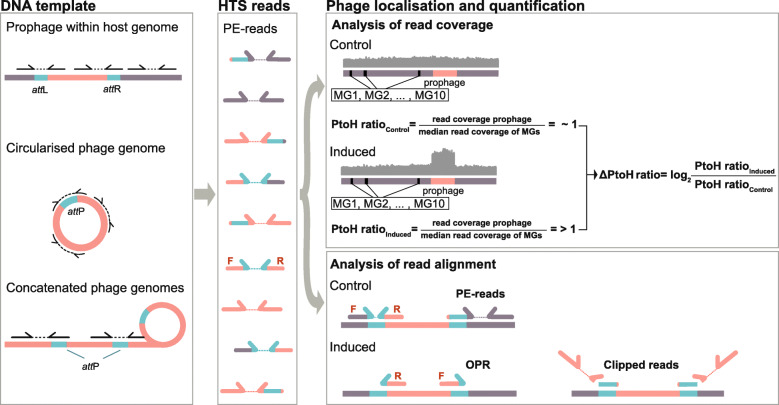
Fig. 2.
Localisation and quantification of inducible prophages by HTS reads. In our study, DNA templates from either integrated prophages (i.e. within host genomes) or induced prophages (i.e. from circularised and concatenated phage genomes) were subjected to HTS. Depending on the type of template DNA, HTS generates paired-end (PE) reads of bacterial (dark purple), viral (red) or combination of both (mixture of dark purple, turquoise and red) origin. Alignment of PE-reads from prophages to the host reference genome is expected to result in an even read coverage and a PtoH ratio around one. PE-reads from circularised and concatenated phage genomes are expected to increase the read coverage at the prophage region compared to the remainder of the reference genome resulting in a PtoH ratio larger than one. The read coverage of the reference genomes was calculated as the median read coverage of 10 universal, single-copy, phylogenetic marker genes (MGs). The ΔPtoH ratio represents the change in the prophage activity between induced and non-induced prophages measured as mean l2fc. Reads originating from regions containing the attL or attR sites of prophages (turquoise) reveal usual PE read characteristics (F-R orientation, standard insert size) when being aligned to the reference genome. However, PE-reads originating from regions containing the attP site of circularised and concatenated phage genomes (turquoise) are outward-oriented (R-F orientation) and have an enlarged insert size (approximating the genome size of the respective phages) when being aligned to reference genomes. These PE-reads are referred to as outward-oriented paired-end reads (OPRs). PE-reads originating from excised prophages and covering the attP site align only partially to the reference genome of the lysogen; thus, they give rise to reads we refer to as clipped reads. The accumulation of clipped reads and OPRs around att sites, and increased ΔPtoH ratio allow to identify and locate inducible prophages