Figure 1.
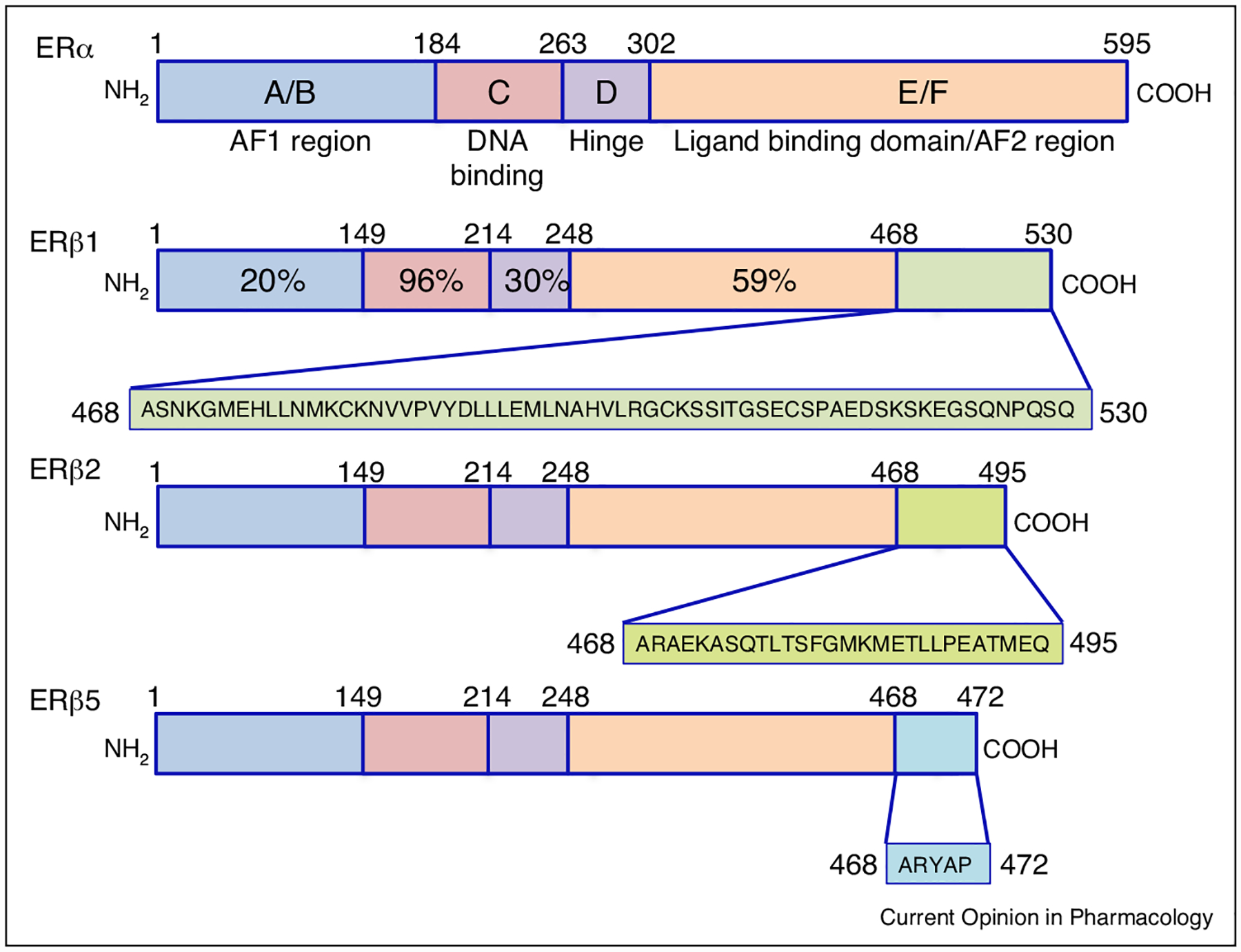
Comparative representation of ERβ isoforms. Each receptor is represented by a colored bar with structural domains conserved across the steroid/thyroid super-family of nuclear receptors indicated. Shading in the carboxyl terminus of each ERβ isoform is representative of the predicted differential amino acid sequences in each receptor isoform as a result of alternative splicing. Percentage on ERβ1 highlights the homology shared between each domain of ERα and ERβ1. The amino-terminal A/B regions have a transactivation domain with ligand-independent function and recruits co-activators and co-repressors. The C region contains the DNA-binding domain (DBD), which is needed for binding to specific estrogen response elements (EREs) in estrogen responsive genes. The D region contains several functional domains, including the hinge domain to connect C and E/F. The carboxy-terminal regions E and F contain the ligand-binding domain, which is needed for ligand binding, receptor dimerization, and nuclear translocation. ERβ variants are identical in the first 468 amino acid (AA) sequences, and divergence of AAs are shown after 468 in different colors and representative boxes underneath the variant protein structure.
