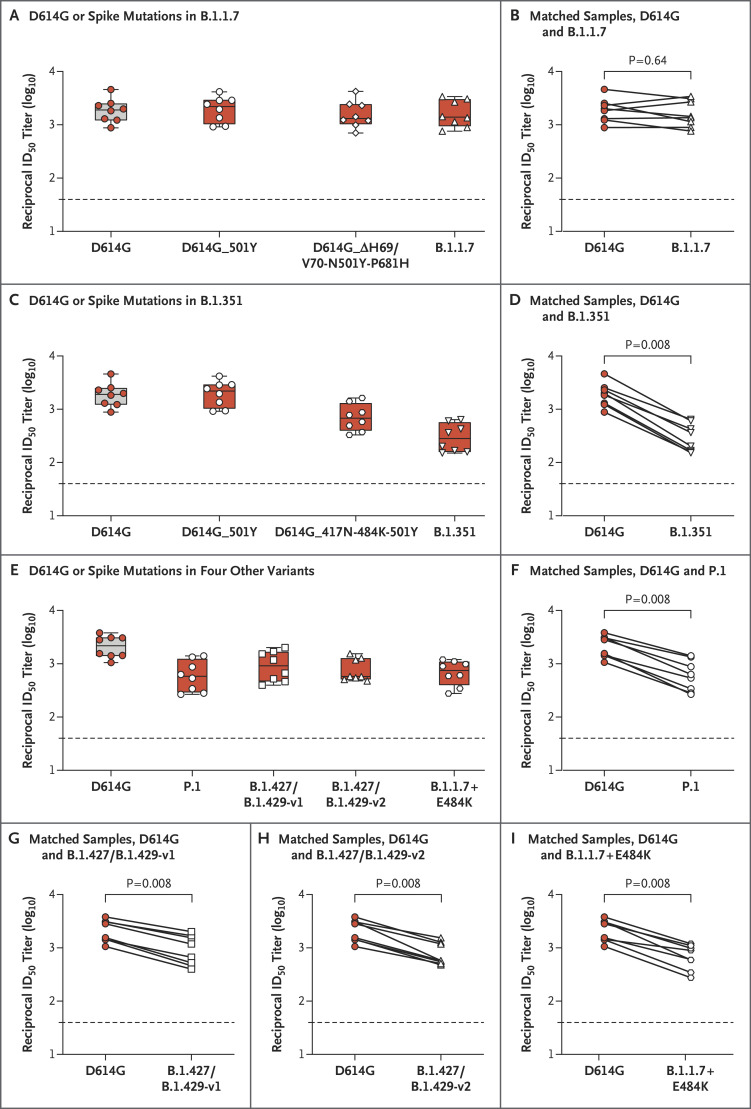
Figure 1. Neutralization of SARS-CoV-2 Pseudoviruses in Serum Samples.
Serum samples obtained from participants who received the mRNA-1273 vaccine in a phase 1 trial were collected on day 36 (7 days after the participants received the second dose of the vaccine). Neutralization was measured with the use of a recombinant vesicular stomatitis virus (rVSV)–based pseudovirus neutralization assay that incorporated D614G or the indicated spike mutations present in the B.1.1.7 variant (Panels A and B), the B.1.351 variant (Panels C and D), or the P.1 variant, the B.1.427/B.1.429 (versions 1 and 2) variants, and the B.1.1.7+E484K variant (Panels E through I). The red dots indicate the results from serum samples of the individual participants; the white dots, white diamonds, and white triangles the same samples tested against the variants shown on the x axis; and the horizontal dashed lines the lower limit of quantification. The reciprocal neutralizing titers on the pseudovirus neutralization assay at a 50% inhibitory dilution (ID50) are shown. In Panels A, C, and E, boxes and horizontal bars denote the interquartile range (IQR) and the median neutralizing titer, respectively. Whisker end points are equal to the maximum and minimum values below or above the median at 1.5 times the IQR. In Panels B, D, F, G, H, and I, the lines connect the D614G and variant neutralization titers in matched samples. We detected reductions by a factor of 1.2 in titers of neutralizing antibodies against the B.1.1.7 variant (Panel B), a factor of 6.4 against the B.1.351 variant (Panel D), a factor of 3.5 against the P.1 variant (Panel F), a factor of 2.3 against the B.1.427/B.1.429-v1 variant (Panel G), a factor of 2.8 against the B.1.427/B.1.429-v2 variant (Panel H), and a factor of 3.1 against the B.1.1.7+E484K variant (Panel I). Statistical analysis of matched pairs was performed with the use of the Wilcoxon signed-rank test.