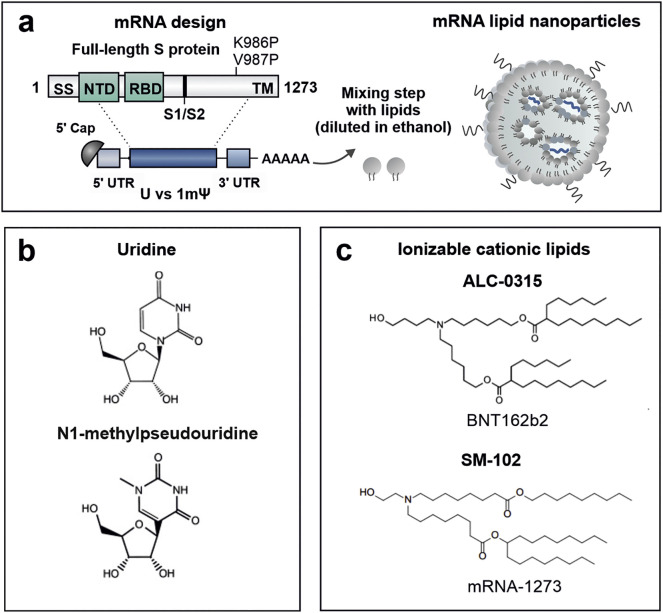
Fig. 2.
COVID-19 mRNA vaccine design. (a) The COVID-19 mRNA vaccines contain a mRNA sequence encoding the full length S protein with two proline substitutions (K986P and V987P). The S protein's genetic code is flanked by structural elements to produce a mature mRNA. Each of these elements can be optimized in order to modulate mRNA stability, translation capacity and innate immune activity. (b) While the CVnCoV vaccine candidate make use of unmodified uridines, BNT162b2 and mRNA-1273 are nucleoside-modified with a substitution of N1-methylpseudouridine (1mψ) for uridine (U). (c) Chemical structures of the ionizable cationic lipids ALC-0315 (((4-Hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate)) and SM-102 (Heptadecan-9-yl 8-((2-hydroxyethyl)(8-(nonyloxy)-8-oxooctyl)amino)octanoate) used in the LNP formulation of BNT162b2 and mRNA-1273, respectively. The ionizable cationic lipid used in CVnCoV has not (yet) been disclosed. Abbreviations: SS; Signal sequence, NTD; N-terminal domain, S1/S2; native furine cleavage site, TM; transmembrane domain.