Abstract
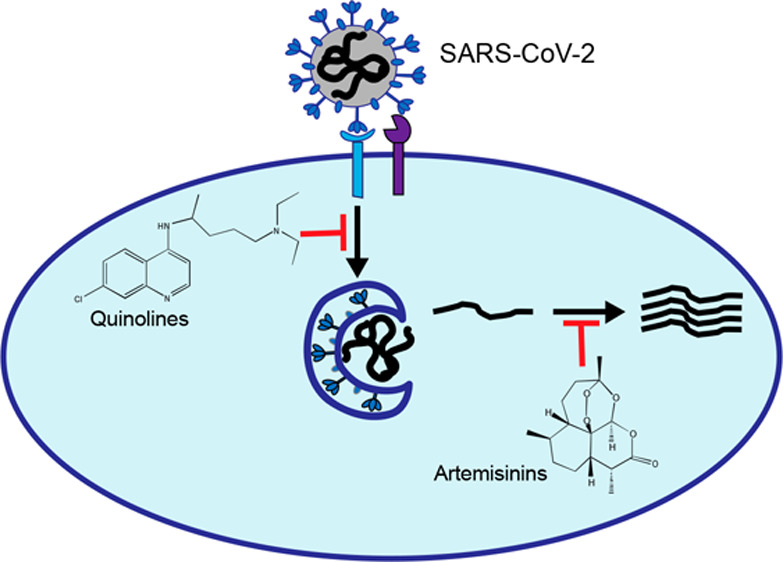
The coronavirus disease-2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 116 million individuals globally and resulted in over 2.5 million deaths since the first report in December 2019. For most of this time, healthcare professionals have had few tools at their disposal. In December 2020, several vaccines that were shown to be highly effective have been granted emergency use authorization (EUA). Despite these remarkable breakthroughs, challenges include vaccine roll-out and implementation, in addition to deeply entrenched antivaccination viewpoints. While vaccines will prevent disease occurrence, infected individuals still need treatment options, and repurposing drugs circumvents the lengthy and costly process of drug development. SARS-CoV-2, like many other enveloped viruses, require the action of host proteases for entry. In addition, this novel virus employs a unique method of cell exit of deacidified lysosomes and exocytosis. Thus, inhibitors of lysosomes or other players in this pathway are good candidates to target SARS-CoV-2. Chemical compounds in the quinoline class are known to be lysomotropic and perturb pH levels. A large number of quinolines are FDA-approved for treatment of inflammatory diseases and antimalarials. Artemisinins are another class of drugs that have been demonstrated to be safe for use in humans and are widely utilized as antimalarials. In this Review, we discuss the use of antimalarial drugs in the class of quinolines and artemisinins, which have been shown to be effective against SARS-CoV-2 in vitro and in vivo, and provide a rationale in employing quinolines as treatment of SARS-CoV-2 in clinical settings.
Keywords: antiviral, antimalarial, SARS-CoV-2, artemisinin, chloroquine, therapeutics, drug repurposing
In December 2019, Wuhan, China reported an unusual pneumonia of unknown origin.1 Clinical features of this pneumonia resembled that of severe acute respiratory virus (SARS).2 Transmission electron microscopy of the virus isolated from patients revealed classical features of coronaviruses, and the viral genome isolated from patients clustered into a clade of betacoronaviruses distinct from SARS-CoV.1,3 Thus, this disease, named coronavirus disease-19 (COVID-19) was caused by a novel coronavirus (2019-nCoV), which was later renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On March 11 2020, COVID-19 was declared a global pandemic by the World Health Organization (WHO).4 The virus spreads primarily through droplets, direct contact, and likely through aerosolization.5−9 Symptoms of SARS-CoV-2 range from mild to severe and likely depend on patient characteristics such as genetics, immune response, and other disease complications. COVID-19 patients may experience dry cough, loss of taste and smell, gastrointestinal symptoms, fatigue, fever, shortness of breath, pneumonia, lung disease characterized by ground-glass opacity upon CT scan, myocardial injury, kidney dysfunction, and death.1,10−12 Severe disease progression is associated with inflammation characterized by increased levels of IL-6, serum ferritin, and C-reactive protein (CRP), increased leucocytes and neutrophils, decreased lymphocytes, platelets, and hemoglobin and concomitant increased bilirubin, increased D-dimer which is a signature of resolved blood clots, and increased alanine aminotransferase, aspartate aminotransferase, and myoglobin, indicative of muscle, heart, liver, and kidney damage.12,13 Even in patients whose SARS-CoV-2 infection has resolved, sequelae affecting the cardiac, pulmonary, and neurologic systems months after recovery are now being documented,11 and further monitoring post recovery will be warranted to measure the magnitude of this disease. In addition, reinfection of previously infected patients suggests that the mounted immune response to infection may confer incomplete protection.14
Current CDC standard of care guidelines for infected patients is limited to infection control, supportive care, and ventilatory support.15 Multiple companies are racing toward producing a safe and effective vaccine. Of these, safety and efficacy data from clinical trials with the ChAdOx1 nCoV-19 vaccine (AZD1222)16 and BNT162b2 vaccine17 have been published. These vaccines have been granted emergency use authorization (EUA), and vaccinations are currently being delivered to people around the world. Concurrently, other vaccine manufacturers including Johnson & Johnson and Novavax are completing clinical trials on their vaccine candidates. While scale-up of manufacturing and roll out and implementation of vaccine strategy have been remarkable, entrenched antivaccination viewpoints and equitable global vaccine access are some of the challenges facing a global mass vaccination strategy. These challenges and the fact that the ongoing pandemic demands a variety of methods to curb infections and deaths provide reason for consideration of antiviral drugs in responding to the pandemic.
It takes an average of 14 years and about $2.6 billion to develop a drug from bench to clinical use.18 Repurposing of FDA-approved drugs allows for use of previously validated drugs for which safety and efficacy profiles are known for ailments beyond the originally approved indication, and it has gained attraction as an inexpensive alternative to drug discovery. The antimalarials chloroquine (CQ) and artemisinin (ART) have been on the market for the last 80 years. CQ is a synthetic derivative of quinine, a compound isolated from the bark of a cinchona tree. Structurally, CQ contains a quinine ring and an amino side chain at the fourth position (Figure 1). CQ has been used as a treatment for malaria since the early 1940s.19 During World War II, it was observed that CQ use in the military was also effective for rheumatoid diseases, inspiring clinical trials using aminoquinolines for diseases like systemic lupus erythematosus (SLE)20−22 and rheumatoid arthritis (RA)23 and is now FDA-approved for treatment of these inflammatory diseases. Quinolines continue to be studied as treatments for various diseases including cancer and viruses.24−26 Currently, artemisinin-based combination therapies (ACT) are recommended by the WHO as first-line therapies for uncomplicated malaria.27 An extract from the sweet wormwood plant Artemisia annua, artemisinin is a sesquiterpene lactone that contains an endoperoxide moiety crucial to its antimalarial activity28 (Figure 2). The activity of artemisinin and its derivatives against other diseases such as cancer29 and viruses30 have been documented. Antimalarials are effective antivirals, hindering viral entry, replication, and release, as well as blocking inflammatory response elicited during viral infections. Over the last 20 years, antimalarials have been repurposed as antivirals, anticancer agents, and treatments for autoimmune disease. Our Review will highlight the ability of antimalarials to interfere with SARS-CoV-2 and the use of antimalarials as treatment options for the COVID-19 pandemic.
Figure 1.
Chemical structures of quinolines. (A) Chloroquine, (B) hydroxychloroquine, (C) monodesethylchloroquine, (D) amodiaquine, (E) monodesethylamodiaquine, (F) ferroquine, (G) mefloquine, and (H) pyronaridine.
Figure 2.
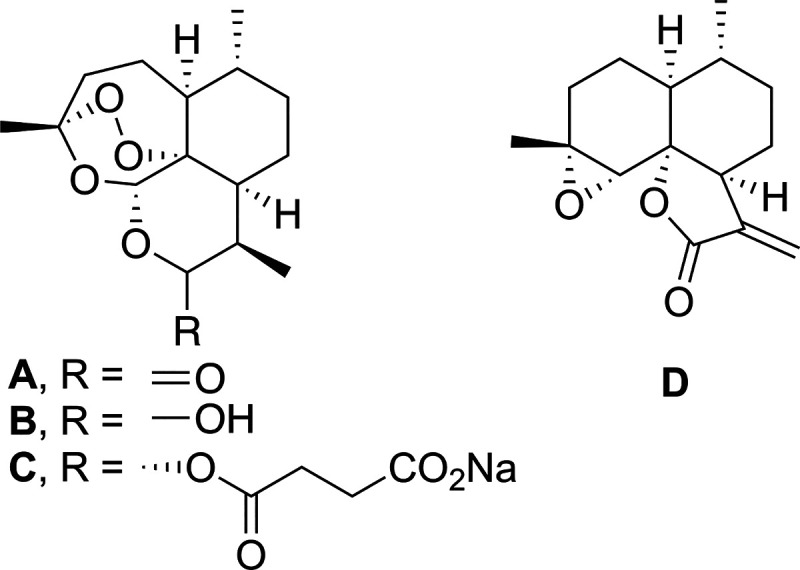
Chemical structures of artemisinin and its derivatives. (A) Artemisinin, (B) dihydroartemisinin, (C) artesunate, and (D) arteannuin B.
SARS-CoV-2 Biology
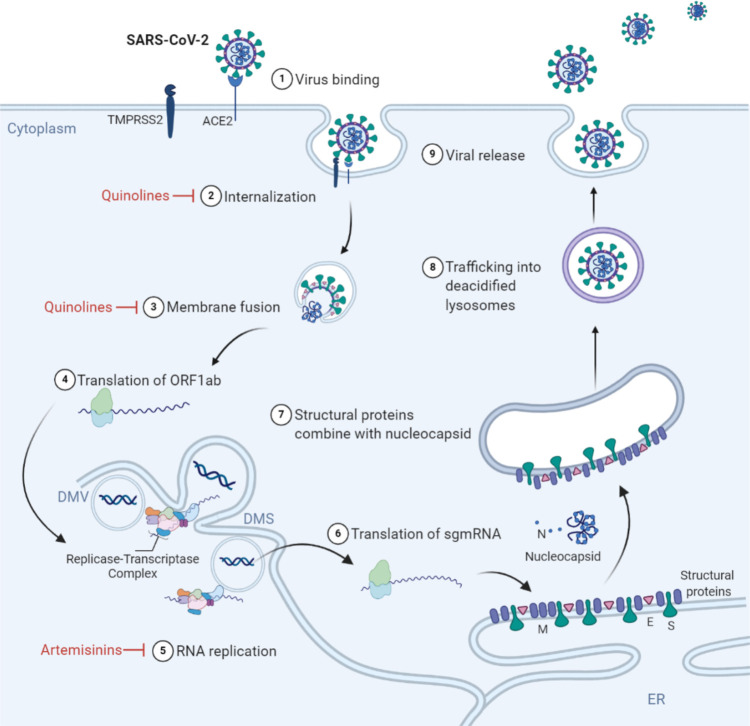
SARS-CoV-2 is a positive-sense, single-stranded RNA betacoronavirus in the Coronaviridae family.1 The virus genome contains up to 16 open reading frames (ORFs), including the N-terminal ORFs 1a and 1b and the C-terminal ORFs which are expressed from subgenomic RNAs (sgRNAs). The viral replicase-transcriptase complex (RTC) is composed of 16 nonstructural proteins (nsPs) which are expressed from the ORF1ab genome transcript, while structural proteins, including spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleoprotein (N), are expressed from sgRNA. Accessory proteins are also expressed from alternate ORFs and have been observed to be dispensable in cell culture infection conditions.31 The S protein, expressed on the surface of the enveloped virion, binds angiotensin converting enzyme 2 (ACE2), leading to receptor-mediated endocytosis.32−34 ACE2 is present, albeit at low levels, on a wide variety of cells such as lung epithelial, alveolar, and intestinal cells.35,36 The wide range of cells permissive to SARS-CoV-2 infection underlies the broad range of symptoms and organs affected following infection. Cellular proteases, such as transmembrane serine protease 2 (TMPRSS2), cathepsin B, and cathepsin L, are required for S protein priming prior to conformational changes triggered by a low pH environment in the endosome.32 Following internalization, coronavirus genome translation and replication occur in the cytoplasm. The viral RTC is initially translated as a polyprotein and processed by encoded proteases for replication and transcription to occur. Perinuclear ER-derived double membrane vesicles (DMVs) and double membrane spherules (DMSs) are formed to serve as sites of viral replication carried out by the RNA-dependent RNA polymerase nsP12 and its cofactors.37 The viral genome, coated with N proteins, buds into the ER and ER-Golgi-intermediate compartment (ERGIC) to be enveloped with host membranes and viral S, E, and M proteins.38 After trafficking to the trans-Golgi network, virions are trafficked to deacidified late endosomes/lysosomes containing inactivated enzymes and bud through a noncanonical lysosomal trafficking pathway.39 The SARS-CoV-2 lifecycle and points of antimalarial interference are indicated in Figure 3.
Figure 3.
Antimalarial compounds inhibit SARS-CoV-2. (1) SARS-CoV-2 spike (S) protein binds host receptor angiotensin converting enzyme 2 (ACE2), leading to (2) viral internalization via receptor-mediated endocytosis. (3) A low pH environment in the endolysosome compartment triggers a conformational change that allows S protein, primed by host proteases transmembrane serine protease 2 (TMPRSS2) or cathepsin B, to mediate membrane fusion, releasing the viral genome into the cytoplasm. (4) The open reading frame 1ab (ORF1ab) is immediately translated by the host cell machinery, which encodes for the nonstructural proteins RNA-dependent RNA polymerase and cofactors that make up the replicase transcriptase complex (RTC). (5) The viral genome is replicated via the RTC in a microenvironment made up of double membrane vesicles (DMV) and double membrane spherules (DMS). (6) Subgenomic mRNA (sg mRNA) encodes for the structural proteins nucleocapsid (N), membrane (M), envelope (E), and spike (S), which are cotranslationally inserted into the endoplasmic reticulum (ER). (7) The N-coated viral genome buds into the endoplasmic reticulum (ER) and ER-Golgi-intermediate compartment (ERGIC) where it is enveloped with M, E, and S proteins. (8) Mature virions are trafficked to deacidified lysosomes, and (9) virions are released through exocytosis. Antimalarials inhibit various steps of the SARS-CoV-2 lifecycle, as shown in red.
Quinolines
The U.S. FDA approves the use of the 4-aminoquinoline chloroquine (CQ) for malaria27 as well as the autoimmune diseases rheumatoid arthritis and systemic lupus erythematosus,40 reflecting this drug’s antiparasitic and anti-inflammatory activities. As a weak base, CQ diffuses by passive transport across membranes toward acidic compartments. There, CQ is protonated and consequently trapped, while the lysosomal compartment is concomitantly deacidifed.41 CQ and other quinolines prevent replication of malaria parasites by interfering with heme detoxification within the parasite digestive vacuole.42 In mammalian cells, CQ interferes with the organization of the Golgi apparatus, endocytosis, and causes lysosomal fusion defects.43 These subcellular compartments are important during viral infection for the release of viral genomes, production and processing of viral proteins, and assembly and release of viral progeny. In particular, by raising the pH of lysosomes, quinolines prevent virus entry by inhibiting the pH-dependent fusion step, as well as deactivating lysosomal proteases. CQ and other quinolines discussed here are depicted in Figure 1.
CQ inhibits SARS-CoV-2 in vitro when added at the time of entry44 or prior to infection.45 In addition to CQ, other quinolines such as hydroxychloroquine (HCQ), ferroquine, monodesethyl amodiaquine (mdAQ), mefloquine (MF), and pyronaridine inhibited SARS-CoV-2 with EC50 values less than 2.5 μM.45 Mechanistically, CQ and HCQ inhibit viral entry of SARS-CoV-2 by interfering with endosome maturation.26 Specifically, about 35% of virions colocalized with the early endosome antigen 1 (EEA1) and less than 2.5% of virions colocalized with the late endosomal-lysosomal protein LAMP1 upon CQ and HCQ treatment. In contrast, in untreated cells, the association with EEAI and LAMP1 was 16% and 35%, respectively.26 Furthermore, chloroquine disrupted terminal glycosylation of ACE2 without affecting ACE2 cell surface expression.46 Given the requirement of ACE2 binding for SARS-CoV-2 infection,32 modification of ACE2 could inhibit virion attachment and subsequent viral entry. Following ACE2 binding, the virus utilizes host serine protease TMPRSS2 and cysteine proteases cathepsins B and L to prime the S protein to mediate entry.32 TMPRSS2 is primarily plasma membrane associated, whereas the cathepsins are located intracellularly and require a low pH for activity. Extracellular priming of the S protein by TMPRSS2 likely allows the virus to bypass the requirement for endosomal proteases. Whether the virus employs multiple routes of entry or if the function of host proteases can complement each other creating redundancy and the resulting implication on cell type specificity is not completely known. Single-cell RNA-sequencing data from multiple tissues obtained from healthy human donors showed that cathepsin B was expressed in 70–90% of ACE2-expressing cells, while TMPRSS2 was not coexpressed in many of the ACE2-expressing cells,36 indicating that cathepsin B may play a major role in S glycoprotein processing post-ACE2 binding. CQ, monodesthyl chloroquine (mdCQ), amodiaquine (AQ), mdAQ, MF, and quinacrine inhibited cathepsin B in vitro.47,48 Altogether, quinolines likely inhibit SARS-CoV-2 entry by affecting the terminal glycosylation of ACE2, inhibiting cathepsin B, and preventing endosome maturation.
There is no consensus on whether CQ and HCQ affect SARS-CoV-2 replication. CQ reduced SARS-CoV-2 viral RNA production and N protein expression in Vero E6 cells.44 In contrast, 2 μg/mL of HCQ, which is the achieved plasma concentration of an 800 mg once daily dose of HCQ sulfate, did not inhibit viral replication nor reduce cytopathic effect in Vero E6 cells.49 In a Syrian hamster model that allows SARS-CoV-2 replication to high titers in the lung leading to bronchopneumonia and lung inflammation, HCQ mildly reduced viral replication in the lungs, but this did not lead to any reduction in lung pathology.50 HCQ treatment did not protect hamsters from contracting the virus from close contact with another infected hamster and did not reduce resulting viral titers.50
CQ also inhibits other coronaviruses. SARS-CoV, responsible for the SARS outbreak in 2003, is inhibited by CQ treatment prior to infection46 or at the time of infection.51 CQ also inhibits viral replication in Vero E6 cells post entry, although higher concentrations are required.46 CQ, HCQ, MF, and AQ inhibited SARS-CoV and Middle Eastern respiratory syndrome coronavirus (MERS-CoV) with low micromolar EC50 value.52 When added at the time of infection, but not post infection, CQ reduced genome equivalents of HCoV-OC43, a coronavirus strain which causes the common cold.53 Interestingly, this study showed that administration of CQ to pregnant mothers prepartum and postpartum prior to inoculation of 5-day-old suckling mice prevented infant death, indicating that the prophylactic antiviral activities of CQ were transferred both transplacentally and via maternal milk.53
Quinolines are also effective against other viruses that utilize endocytic pathways for entry into the host cell. AQ, mdAQ, CQ, and mdCQ inhibited early entry events of ZIKV without interfering with virion attachment54 or RNA synthesis.55 These effects were recapitulated in vivo, as suckling mice infected with ZIKV were more likely to survive if the pups were nursed by CQ-treatment mothers.55 Of note, AQ inhibited viruses that require endosomal acidification such as EBOV, SARS-CoV, Venezuelan equine encephalitis virus, Rabies virus, Junin virus, and Chikungunya virus, but not those that enter in a pH-independent manner such as poliovirus and human cytomegalovirus.47,56,57
Betacoronaviruses including SARS-CoV-2 exit from cells by a unique method of exocytosis from deacidified lysosomes, which also alters antigen presentation and immune system activation following infection.39 Lysosomotropic quinolines, which also deacidify lysosomes, could promote viral exit and spread and/or inhibit recognition by the host immune system. Further studies are required to fully elucidate the mechanism of host cell lysosome deacidification by betacoronaviruses, as well as the effect of quinolines at the later stages of infection.
These studies show quinolines are most effective against SARS-CoV-2 and other coronaviruses that enter host cells via a pH-dependent mechanism when administered prior to or at the time of infection. Thus, these therapeutics may best be used as a prophylactic treatment.
Artemisinins
Artemisinin is a sesquiterpene lactone derived from the sweet wormwood plant Artemisia annua. The potency of artemisinin against hemoglobin-digesting parasites is attributable to heme-mediated cleavage of the endoperoxide bond leading to reactive carbon-centered radicals and parasite death.58 Artemisinin also demonstrates activity against viruses and cancer cells, albeit at higher concentrations than when used as an antiparasitic agent; this activity is attributed to the compound’s pleiotropic effects on the cell. Artemisinin and derivatives discussed here are depicted in Figure 2.
Artemisinin and seven derivatives were examined for their activity against SARS-CoV-2 in Vero E6 cells by quantifying viral RNA in the supernatant by qRT-PCR after 24 h of treatment.59 Lumefantrine (LF), a partner drug with artemether in artemisinin-based combination therapies (ACT) for treatment of malaria, was also included. The three most potent artemisinin derivatives against SARS-CoV-2 were arteannuin B (artB), artesunate (AS), and dihydroartemisinin (DHA) with EC50 values of 10–14 μM, while LF demonstrated an EC50 of 23 μM.59 When added post entry but not at the time of infection, artB and LF inhibited viral RNA recovered in the supernatant, as well as viral N protein production visualized by IFA and Western blot,59 indicating that these compounds affected viral replication downstream of early entry events. In a separate study, the antiviral effects of five ACTs, namely, (1) MF-DHA, (2) mdAQ-DHA, (3) pyronaridine-DHA, (4) LF-DHA, and (5) piperaquine-DHA were investigated.60 ACTs were added to Vero E6 cells at concentrations relative to maximum blood concentration (Cmax) for 4 h prior to SARS-CoV-2 infection of Vero E6 cells, then viral RNA was assayed by qRT-PCR. The two best combinations were MF-DHA and mdAQ-DHA, which inhibited SARS-CoV-2 with 99.6% and 85.8% efficiency at 2 × Cmax, which dropped to 72% and 32% inhibition at 1 × Cmax, respectively.60 The efficacy of these two combinations is likely due to the effects of MF and mdAQ on viral entry, as described earlier, due to the timing of treatment in this study. In a small 41-person study, ACTs were shown to be therapeutic in SARS-CoV-2-positive patients.61 The administration of artemisinin-piperaquine (ART-PQP) reduced viral titers to undetectable levels in all 23 patients by day 21, whereas patients that did not receive ART-PQP treatment only cleared virus at day 36.61 A major caveat of this study is that patients in each group also received multiple other treatments, including interferon α-1b, HCQ, ribavirin, oseltamivir, lopinavir, and other herbal remedies.61
Treatment of COVID-19 Patients with Antimalarials
The use of quinolines to treat COVID-19 patients is controversial. Data obtained from hospitalized patients from several clinical trials using CQ or HCQ have been released. Because of the lack of treatment options and urgency of the COVID-19 pandemic, many of the following reports are observational results of treated patients rather than randomized clinical trials. A handful of reports, some of which examined a large cohort of patients, show improvements in mortality when treated with HCQ. Randomized clinical trials show that HCQ does not improve mortality but could improve lung pathology. Lastly, randomized clinical trials on nonhospitalized participants that are SARS-CoV-2 PCR-negative show no benefit as prophylaxis. It is worth examining the differences in the way various studies are conducted to understand the differences in outcomes.
Observational Studies Showing HCQ Benefit
The first few studies that were published prioritized getting out an important novel message, deprioritized study size, and did not control for confounding factors. Published in March 2020, data from Marseille, France, suggested that HCQ reduces detection of virus.62 Of the 14 patients who received HCQ, 8 tested PCR-negative for SARS-CoV-2 on day 6 of treatment, in contrast to 2 out of the 16 patients not receiving treatment. All six of the patients who received a combination therapy of HCQ and azithromycin (AZT) were PCR-negative from nasopharyngeal samples.62 There are some major caveats to the general findings, including a small sample size, nonrandomized study, and the patients in untreated arms were cared for in a different hospital or refused treatment. For further insight, the reader is referred to consideration of this work in refs (63) and (64). In contrast to these results, analysis of 11 patients in a Parisian hospital given a HCQ and AZT regimen, as reported in ref (62), showed that 5–6 days after treatment, 8 of 10 patients remained positive for SARS-CoV-2 RNA (in this 11-person cohort, one patient died within 5 days of treatment).65
Three large studies showed that HCQ demonstrated a benefit in patient mortality, and studies in which physicians treated patients aggressively and early in disease onset showed a more pronounced reduction in mortality.66−68 Of note, in the study conducted in Michigan, United States66 physicians followed protocols that were standardized across the six hospitals, giving these results more weight. Here, HCQ treatment reduced in-hospital mortality of SARS-CoV-2 PCR-positive patients.66 Of the 2541 patients, 1202 were treated with HCQ, 147 were treated with AZT, 783 were given a combination therapy of HCQ and AZT, and 409 were not treated with either drug. Drug treatment was given to 82% patients within 24 h of admission and 91% within 48 h of admission. Patients received aggressive early medical intervention, and HCQ was delivered in conjunction with serial QTc checks. A QTc interval-based algorithm to ensure safe administration, and medical care across the different hospitals were administered in a coordinated manner with standardized procedures. In this setting, HCQ treatment decreased the mortality hazard ratio by 66% compared with untreated patients, and the addition of AZT decreased the mortality hazard ratio by 71%. Of note, 68% of patients also received corticosteroid treatment of methylprednisone and/or prednisone, but corticosteroid treatment showed no association with mortality.
The second large study showing that HCQ treatment improved mortality is a retrospective analysis of hospitalized SARS-CoV-2 PCR-positive patients in 33 clinical centers throughout Italy conducted by the COVID-19 RISK and Treatments (CORIST) Collaboration.67 Of a total 3451 patient outcomes analyzed, 817 did not receive HCQ, while the majority of the 2634 individuals who were treated with HCQ for 5–15 days received the drug within 24 h and the rest received it within 2–3 days. Patients receiving HCQ were more likely to be young men with high CRP and less likely to have heart disease, kidney disease, or cancer stage 3a or greater. Patients who received HCQ also received antivirals or corticosteroids. With these caveats, there was a 9% death rate in HCQ-treated patients compared with 16% in non-HCQ-treated patients. Adjusting for age, preexisting conditions, additional treatments received, and different hospital settings, patients receiving HCQ showed a 30% reduced risk of death. In Italy, HCQ treatment is recommended even for patients with mild pneumonia, suggesting that HCQ treatment early in the course of disease is crucial. The benefit of HCQ was heightened in cases with high CRP, indicating that the anti-inflammatory effect could be particularly beneficial.
Lastly, a study on 8075 patients from 109 Belgian hospitals, most of whom were severely ill, showed that HCQ treatment decreased mortality rates.68 In the study, 4542 patients were treated with HCQ, 78% of which were dosed within 24 h, while 3533 patients were not given HCQ treatment. Those given HCQ treatment were more likely to be younger, male, and have fewer comorbidities such as cardiovascular disease, renal disease, cancer, obesity, and history of smoking, but they tended to be more severely ill with COVID-19 as evidenced by radiological pneumonia, acute respiratory distress syndrome (ARDS), ICU transfer, and necessity of ventilatory support within 24 h of hospital admittance. Adjusting for age, sex, and preexisting conditions, the direct-adjusted mortality at 40 days post-treatment was 19% when patients were given HCQ versus 26.5% when given supportive care.
Observational Studies Showing No HCQ Benefit
In contrast, other groups report no benefit of HCQ administration on patient survival. Two large studies of patients in New York City, United States, found no benefit of HCQ on survival.69,70 It is important to note that in the New York hospitals, HCQ was recommended only for patients with severe pneumonia and ARDS. The first study is an analysis from one New York City hospital of 1376 patients who tested positive for SARS-CoV-2.69 Of the 811 patients treated with HCQ and who were more severely ill at baseline, 373 received treatment within 24 h of presentation to the emergency department, while 697 were treated within 48 h. There was no significant association between HCQ treatment and the end point of intubation or death. Of note, this study adjusted for factors such as predictors of respiratory failure, probability of receiving HCQ treatment, and handling of missing data.69
The second study randomly selected 1438 patients out of a sample of 7914 patients with COVID-19 admitted to 25 metropolitan New York hospitals.70 Patients receiving HCQ or a combination of HCQ and AZT presented with more severe clinical illness as determined by chest imaging, respiratory rate, oxygen saturation, and hepatic measurements of alanine aminotransferase and aspartate aminotransferase levels, in addition to being more likely to have chronic lung disease and cardiovascular conditions. Medication was administered at any time during hospitalization. Adjusted for demographics, specific hospital, preexisting conditions, and illness severity, treatment with HCQ, AZT, or a combination of HCQ and AZT did not improve survival outcomes when compared with patients that did not receive either of these drugs. Importantly, cardiac arrest was more frequent in patients treated with HCQ.70
An observational study of 3325 SARS-CoV-2 PCR-positive patients admitted to 13 hospitals in New Jersey, United States, did not find a survival benefit associated with HCQ treatment.71 Patients positive for SARS-CoV-2 requiring oxygen but not intensive care did not show improved outcomes with HCQ treatment.72 Here, the primary outcome was defined as survival without transfer to an intensive care unit at 21 days, and secondary outcomes were defined as survival without acute respiratory distress syndrome, weaning off of oxygen, and discharge from the hospital at day 21. Lastly, a retrospective study of patients with rheumatic conditions found no association of HCQ treatment with the prevention of SARS-CoV-2 infection.73
Randomized Clinical Trials Showing HCQ Benefit
In addition to observational studies, several randomized clinical trials with HCQ have been conducted.74−77 Published in April 2020, early in the pandemic, data from a small 22-person randomized clinical trial in China suggested a mild improvement in patient outcomes with chloroquine treatment.74 Here, patients who tested PCR-positive for SARS-CoV-2 were treated with CQ or a combination of lopinavir and ritonavir. In both treatment arms, there was no significant difference in SARS-CoV-2 PCR positivity or T-cell counts. However, patients who received CQ showed slight improvements in lung pathology upon CT scan and were discharged at a slightly earlier time point post-treatment.74
Randomized Clinical Trials Showing No HCQ Benefit
In contrast to the small study discussed above, other larger studies showed no HCQ benefit. In Brazil, a randomized clinical trial of 667 patients, of which 504 were confirmed to have COVID-19 by PCR positivity, was conducted.75 In roughly a 1:1:1 random assignment, 217 patients were treated with a combination of HCQ and AZT, 221 patients were treated with HCQ, and 229 patients received neither drug but received glucocorticoids, immunomodulators, antibiotics, or antivirals at the discretion of the treating physician.75 Patients in this study were classified to have mild to moderate disease and would be excluded if receiving supplemental oxygen more than 4L/min by nasal cannula, 40% by Venturi mask, high-flow nasal canula, invasive or noninvasive ventilation, or prolonged QTc interval on electrocardiograms. After 15 days of treatment, patients were scored on a seven-level ordinal scale from no hospitalization to death. There was no significant difference in outcomes in of patients given HCQ, HCQ + AZT, or other types of treatment, but there were more incidences of prolonged QTc intervals in HCQ-treated patients.
In the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial, 1561 patients were assigned HCQ treatment and 3155 assigned standard of care in a 1:2 ratio in 176 hospitals across the United Kingdom.76 At day 28, there was a slight 1.09 increased rate ratio in mortality of HCQ treated patients, with a 27% mortality rate in HCQ-treated vs 25% mortality rate in non-HCQ-treated patients, and a slightly lower discharge rate of HCQ-treated (59.6%) vs non-HCQ-treated patients (62.9%). Of note, there was no difference in death rate ascribed to COVID, but rather the slight increase in deaths were due to cardiac causes and non-SARS-CoV-2 infection.
The WHO Solidarity Trial Consortium, which spanned 405 hospitals in 30 countries, tested the use of remdesivir, HCQ, lopinavir, interferon beta-1a, or standard of care in treatment of COVID-19 patients,77 and we will focus on HCQ. Patients were randomly assigned in a 1:1 ratio the drug treatment available or to local standard of care. Within 28 days, 104 of 947 patients treated with HCQ died vs 84 of 906 non-HCQ-treated patients, demonstrating a 1.19 rate ratio. None of the drugs tested reduced mortality, initiation of ventilation usage, or discharge rates.77
Randomized Clinical Trials Showing No HCQ Benefit as Prophylaxis
A small randomized clinical trial of nonsymptomatic healthcareworkers at two hospitals in Pennsylvania were assigned in a 1:1 ratio to HCQ or placebo to examine if HCQ prophylaxis taken daily for 8 weeks would prevent SARS-CoV-2 infection.78 Four of 64 HCQ-treated and 4 of 61 placebo-treated participants tested positive via a nasopharyngeal swab, thus indicating that there was no prophylactic effect of HCQ on SARS-CoV-2 infection.
A randomized double-blind study in Minneapolis, United States, and Montreal, Canada, was conducted to test HCQ as postexposure prophylaxis.79 Participants, who were asymptomatic, were enrolled if they were exposed to a confirmed COVID-19 individual. Within 4 days of exposure, 414 participants were randomly assigned to receive HCQ, and 407 received placebo folate treatment for 5 days. They were then observed at days 5, 10, and 14 for symptoms compatible with the disease within 14 days or for confirmation with SARS-CoV-2 PCR positivity. There was no difference in between treatment groups in outcomes at days 5 and 10. At day 14, there was a slight increase in COVID-19 cases in the placebo-treated group, although this was not statistically significant. No serious adverse effects were observed with HCQ treatment. These studies are summarized in Table 1.
Table 1. Summary of HCQ Treatment in Observational Studies and Randomized Clinical Trialsa.
| study
design |
therapeutic treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| no. | RCT | N | no HCQ | HCQ | patient disease severity at start of study | measured outcome | HCQ benefit | reference | country |
| 1 | no | small | SOC | 200 mg 3×/day for 10 days | mild | PCR-negative at D6 | yes | Gautret et al.62 | France |
| 2 | no | large | SOC: anti-inflammatories | 400 mg 2×/day for 2 doses on D1 and 200 mg 2×/day D2–5 + SOC | mild | survival | yes | Arshad et al.66 | United States |
| 3 | no | large | SOC: antivirals, anti-inflammatories | 400–600 mg/day for 5–15 days + SOC | mild | survival | yes | Di Castelnuovo et al.67 | Italy |
| 4 | no | large | SOC | 2400 mg over 5 days | severe | survival | yes | Catteau et al.68 | Belgium |
| 5 | no | small | N/A | 200 mg 3×/day for 10 days | moderate | PCR-negative at D6 | no | Molina et al.65 | France |
| 6 | no | large | SOC: antivirals, anti-inflammatories, antibiotics, ACE inhibitors, ARBs | 600 mg 2× on D1, then 400 mg/day D2–5 + SOC | moderate to severe | survival | no | Geleris et al.69 | United States |
| 7 | no | large | SOC: antivirals, anti-inflammatories, antibiotics, ACE inhibitors, ARBs | 200–600 mg 1–2×/day | mild to moderate | survival | no | Rosenberg et al.70 | United States |
| 8 | no | large | SOC: antivirals, anti-inflammatories, antibiotics, ACE inhibitors, ARBs | 800 mg 1×/day for D1, then 400 mg 1×/day for D2–5 | mild to moderate | survival | no | Ip et al.71 | United States |
| 9 | no | medium | SOC | 600 mg/day | severe | survival without transfer to ICU at D21 | no | Mahevas et al.72 | France |
| 10 | no | large | N/A | 400 mg/day | asymptomatic | COVID-19 symptom onset | no | Gentry et al.73 | United States |
| 11 | yes | small | antivirals | 500 mg 2×/day for 10 days (CQ) | moderate to severe | lung pathology | yes | Huang et al.74 | China |
| 12 | yes | large | SOC: antivirals, anti-inflammatories, antibiotics | 400 mg 2×/day for 7 days + SOC | mild to moderate | COVID-19 progression at D15 | no | Cavalcanti et al.75 | Brazil |
| 13 | yes | large | SOC | 800 mg at 0 and 6 h, then 400 mg 2×/day for 9 days + SOC | moderate to severe | survival | no | Horby et al.76 | United Kingdom |
| 14 | yes | large | local SOC | 800 mg at 0 and 6 h, then 400 mg 2×/day for 10 days + SOC | moderate to severe | survival | no | Pan et al.77 | 30 countries |
| 15 | yes | small | microcrystalline cellulose tablets | 600 mg 1×/day with food | asmptomatic | PCR-positive within 8 weeks | no | Abella et al.78 | United States |
| 16 | yes | large | placebo folate tablets | 800 mg at 0 h, 600 mg at 6–8 h, then 600 mg/day for 4 days | asymptomatic | COVID-19 symptom onset | no | Boulware et al.79 | United States and Canada |
RCT: randomized clinical trial, N = number of participants in study, small <100, medium >100 and <500, large >500; SOC: standard of care; HCQ: hydroxychloroquine; CQ: chloroquine; ACE inhibitors: angiotensin converting enzyme inhibitors; ARB: angiotensin-receptor blocker; D1: day 1; N/A: not applicable.
Final Thoughts
As of March 5, 2021, over 116 million individuals have been infected with SARS-CoV-2 globally resulting in more than 2.5 million deaths.80 In the United States alone, there have been close to 30 million infections (∼9% of the U.S. population) and over half a million deaths.80 On December 11, the FDA approved emergency use authorization for the first vaccine in the COVID-19 pandemic. The vaccine, BNT162b2, created by Pfizer and BioNTech was shown to be 95% effective in protecting recipients from symptomatic COVID-19 infection.17 This unprecedented feat, both in rapidity of vaccine development and governmental approval, is promising. However, treatment options following infection are still needed as vaccine production and distribution have been noted to be a bottleneck in the implementation of a vaccination program. Also, reported data was obtained from a clinical trial for individuals aged 16 and above, with no representation of younger, pregnant, or immunocompromised individuals. Finally, the long-term protection offered by the vaccine is currently unknown, as the available safety and efficacy data were obtained two months following the administration of the vaccine.17 These factors contribute to a need for continued work in identifying and testing new and established drugs for the treatment of COVID-19. The advantage of repurposing FDA-approved drugs is the decreased time in obtaining emergency use authorizations, as approved drugs have passed the safety and efficacy tests required in the approval process. Also, for these drugs a dosing regimen is usually established, and pharmacokinetic properties are usually known, providing a baseline for administration in the clinic.
Additional studies are required to fully understand the nuances of SARS-CoV-2 infection. It is currently unknown if there are SARS-CoV-2 receptors other than ACE2, and if so, how many different cellular ligands can act as viral attachment factors or receptors, and are these simultaneously, sequentially, or differentially engaged. It is worth exploring if cathepsins B/L and TMPRSS2 are complementary, if differential expression of these proteases allow cell-specific viral entry, and whether there are other proteases that can serve in this role. A deeper understanding allows more precise drug targeting.
The antimalarials discussed in this Review have been in use in the clinic for several years with established protocols for use and known side effects. As an added benefit, some of these drugs have also been shown to have antiviral effects. A global consensus on the therapeutic benefit of CQ or HCQ administration to COVID-19 patients has not been reached. Randomized clinical trials do not show a benefit or harm of HCQ treatment, and because of these results and possible cardiac complications, some clinicians decline to use these drugs to treat COVID-19. However, several observational clinical studies show that under properly controlled circumstances, quinolines provide a marked benefit in mortality. Given the large numbers of people infected by SARS-CoV-2, drugs showing a possibility of improved mortality outcomes are worth taking into consideration. Hence, while the United States no longer recommends HCQ as a therapeutic, CQ is recommended for treatment of COVID in China.81 It is important to note that in cell-culture-based studies, these drugs were shown to inhibit replication and to function prior to infection or at early times post infection with little to no toxicity, suggesting their use as prophylactics or early intervention strategies. In addition to upcoming vaccine candidates, use of “off the shelf” antimalarials could be explored as a viable treatment option against COVID-19.
Acknowledgments
Funding for this work was provided by NIH R21 AI137900 (C.L.N.) and NIH R21 AI140026 (S.P.R.). C.L.N. and S.P.R. gratefully acknowledge their respective UNMC Start-up Funds and UNMC Diversity Fund Grants. We thank Dr. Jonathan L. Vennerstrom (UNMC) for helpful discussions. Figures 1 and 2 were drawn using ChemDraw version 20.0 and Adobe Illustrator version 24.1 software. Figure 3 was drawn using tools in Biorender.com.
The authors declare no competing financial interest.
This article is made available via the ACS COVID-19 subset for unrestricted RESEARCH re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for the duration of the World Health Organization (WHO) declaration of COVID-19 as a global pandemic.
References
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; Niu P.; Zhan F.; Ma X.; Wang D.; Xu W.; Wu G.; Gao G. F.; Tan W.; (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J. Med. 382 (8), 727–733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; Wang Y.; Li X.; Ren L.; Zhao J.; Hu Y.; Zhang L.; Fan G.; Xu J.; Gu X.; Cheng Z.; Yu T.; Xia J.; Wei Y.; Wu W.; Xie X.; Yin W.; Li H.; Liu M.; Xiao Y.; Gao H.; Guo L.; Xie J.; Wang G.; Jiang R.; Gao Z.; Jin Q.; Wang J.; Cao B. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R.; Zhao X.; Li J.; Niu P.; Yang B.; Wu H.; Wang W.; Song H.; Huang B.; Zhu N.; Bi Y.; Ma X.; Zhan F.; Wang L.; Hu T.; Zhou H.; Hu Z.; Zhou W.; Zhao L.; Chen J.; Meng Y.; Wang J.; Lin Y.; Yuan J.; Xie Z.; Ma J.; Liu W. J.; Wang D.; Xu W.; Holmes E. C.; Gao G. F.; Wu G.; Chen W.; Shi W.; Tan W. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224), 565–574. 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Timeline: WHO’s COVID-19 response. 2020. See the following: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline.
- Liu Y.; Ning Z.; Chen Y.; Guo M.; Liu Y.; Gali N. K.; Sun L.; Duan Y.; Cai J.; Westerdahl D.; Liu X.; Xu K.; Ho K. F.; Kan H.; Fu Q.; Lan K. (2020) Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582 (7813), 557–560. 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Meselson M. (2020) Droplets and aerosols in the transmission of SARS-CoV-2. N. Engl. J. Med. 382 (21), 2063. 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J. L.; Rivera D. N.; Herrera V. L.; Morwitzer M. J.; Creager H. M.; Santarpia G. W.; Crown K. K.; Brett-Major D. M.; Schnaubelt E. R.; Broadhurst M. J.; Lawler J. V.; Reid S. P.; Lowe J. J. (2020) Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 10 (1), 12732. 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen K.; Krambrich J.; Akaberi D.; Hoffman T.; Ling J.; Lundkvist A.; Svensson L.; Salaneck E. (2020) Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci. Rep. 10 (1), 19589. 10.1038/s41598-020-76442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.; Mao Y.; Jones R. M.; Tan Q.; Ji J. S.; Li N.; Shen J.; Lv Y.; Pan L.; Ding P.; Wang X.; Wang Y.; MacIntyre C. R.; Shi X. (2020) Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 144, 106039. 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.; Wang B.; Yuan T.; Chen X.; Ao Y.; Fitzpatrick T.; Li P.; Zhou Y.; Lin Y. F.; Duan Q.; Luo G.; Fan S.; Lu Y.; Feng A.; Zhan Y.; Liang B.; Cai W.; Zhang L.; Du X.; Li L.; Shu Y.; Zou H. (2020) Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 80 (6), 656–665. 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio C.; Collins L. F.; Malani P. (2020) Long-term health consequences of COVID-19. JAMA 324, 1723. 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N.; Zhou M.; Dong X.; Qu J.; Gong F.; Han Y.; Qiu Y.; Wang J.; Liu Y.; Wei Y.; Xia J.; Yu T.; Zhang X.; Zhang L. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 (10223), 507–513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Hu B.; Hu C.; Zhu F.; Liu X.; Zhang J.; Wang B.; Xiang H.; Cheng Z.; Xiong Y.; Zhao Y.; Li Y.; Wang X.; Peng Z. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323 (11), 1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett R. L.; Sevinsky J. R.; Hartley P. D.; Kerwin H.; Crawford N.; Gorzalski A.; Laverdure C.; Verma S. C.; Rossetto C. C.; Jackson D.; Farrell M. J.; Van Hooser S.; Pandori M. (2020) Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect. Dis. 21, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Clinical care guidance for healthcare professionals about coronavirus (COVID-19). 2020. See the following: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html.
- Voysey M.; Clemens S. A. C.; Madhi S. A.; Weckx L. Y.; Folegatti P. M.; Aley P. K.; Angus B.; Baillie V. L.; Barnabas S. L.; Bhorat Q. E.; Bibi S.; Briner C.; Cicconi P.; Collins A. M.; Colin-Jones R.; Cutland C. L.; Darton T. C.; Dheda K.; Duncan C. J. A.; Emary K. R. W.; Ewer K. J.; Fairlie L.; Faust S. N.; Feng S.; Ferreira D. M.; Finn A.; Goodman A. L.; Green C. M.; Green C. A.; Heath P. T.; Hill C.; Hill H.; Hirsch I.; Hodgson S. H. C.; Izu A.; Jackson S.; Jenkin D.; Joe C. C. D.; Kerridge S.; Koen A.; Kwatra G.; Lazarus R.; Lawrie A. M.; Lelliott A.; Libri V.; Lillie P. J.; Mallory R.; Mendes A. V. A.; Milan E. P.; Minassian A. M.; McGregor A.; Morrison H.; Mujadidi Y. F.; Nana A.; O’Reilly P. J.; Padayachee S. D.; Pittella A.; Plested E.; Pollock K. M.; Ramasamy M. N.; Rhead S.; Schwarzbold A. V.; Singh N.; Smith A.; Song R.; Snape M. D.; Sprinz E.; Sutherland R. K.; Tarrant R.; Thomson E. C.; Torok M. E.; Toshner M.; Turner D. P. J.; Vekemans J.; Villafana T. L.; Watson M. E. E.; Williams C. J.; Douglas A. D.; Hill A. V. S.; Lambe T.; Gilbert S. C.; Pollard A. J.; Oxford C. V. T. G. (2020) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J. Med. 383, 2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMasi J. A.; Grabowski H. G.; Hansen R. W. (2016) Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ 47, 20–33. 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Loeb R. F.; Clark W. M.; Coatney G. R.; Coggeshall L. T.; Dieuaide F. R.; Dochez A. R.; Hakansson E. G.; Marshall E. K. Jr.; Marvel C. S.; R M. O.; Sapero J. J.; Sebrell W. H.; Shannon J. A.; Carden G. A. Jr. (1946) Activity of a new antimalarial agent, chloroquine (SN 7618). J. Am. Med. Assoc 130, 1069. 10.1001/jama.1946.02870160015006. [DOI] [PubMed] [Google Scholar]
- Page F. (1951) Treatment of lupus erythematosus with mepacrine. Lancet 258 (6687), 755–758. 10.1016/S0140-6736(51)91643-1. [DOI] [PubMed] [Google Scholar]
- Goldman L.; Cole D. P.; Preston R. H. (1953) Chloroquine diphosphate in treatment of discoid lupus erythematosus. J. Am. Med. Assoc 152 (15), 1428–9. 10.1001/jama.1953.63690150002009a. [DOI] [PubMed] [Google Scholar]
- Kuznik A.; Bencina M.; Svajger U.; Jeras M.; Rozman B.; Jerala R. (2011) Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186 (8), 4794–804. 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- Popert A. J.; Meijers K. A.; Sharp J.; Bier F. (1961) Chloroquine diphosphate in rheumatoid arthritis: a controlled trial. Ann. Rheum. Dis. 20, 18–35. 10.1136/ard.20.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. C.; Ekins S.; Williams A. J.; Tropsha A. (2018) A bibliometric review of drug repurposing. Drug Discovery Today 23 (3), 661–672. 10.1016/j.drudis.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone B. A.; Murthy P.; Miller-Ocuin J.; Doerfler W. R.; Ellis J. T.; Liang X.; Ross M. A.; Wallace C. T.; Sperry J. L.; Lotze M. T.; Neal M. D.; Zeh H. J. 3rd (2018) Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 18 (1), 678. 10.1186/s12885-018-4584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Cao R.; Xu M.; Wang X.; Zhang H.; Hu H.; Li Y.; Hu Z.; Zhong W.; Wang M. (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6, 16. 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Malaria Report; WHO, 2020. See the following: https://www.who.int/publications/i/item/9789240015791.
- Kaiser M.; Wittlin S.; Nehrbass-Stuedli A.; Dong Y.; Wang X.; Hemphill A.; Matile H.; Brun R.; Vennerstrom J. L. (2007) Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160). Antimicrob. Agents Chemother. 51 (8), 2991–3. 10.1128/AAC.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Bu S.; Sun J.; Guo Y.; Lai D. (2018) Artemisinin derivatives inhibit epithelial ovarian cancer cells via autophagy-mediated cell cycle arrest. Acta Biochim Biophys Sin (Shanghai) 50 (12), 1227–1235. 10.1093/abbs/gmy125. [DOI] [PubMed] [Google Scholar]
- Efferth T.; Marschall M.; Wang X.; Huong S. M.; Hauber I.; Olbrich A.; Kronschnabl M.; Stamminger T.; Huang E. S. (2002) Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. (Heidelberg, Ger.) 80 (4), 233–42. 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- Wu F.; Zhao S.; Yu B.; Chen Y. M.; Wang W.; Song Z. G.; Hu Y.; Tao Z. W.; Tian J. H.; Pei Y. Y.; Yuan M. L.; Zhang Y. L.; Dai F. H.; Liu Y.; Wang Q. M.; Zheng J. J.; Xu L.; Holmes E. C.; Zhang Y. Z. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579 (7798), 265–269. 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Kruger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N. H.; Nitsche A.; Muller M. A.; Drosten C.; Pohlmann S. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2), 271. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X.; Liu Y.; Lei X.; Li P.; Mi D.; Ren L.; Guo L.; Guo R.; Chen T.; Hu J.; Xiang Z.; Mu Z.; Chen X.; Chen J.; Hu K.; Jin Q.; Wang J.; Qian Z. (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11 (1), 1620. 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J.; Wan Y.; Luo C.; Ye G.; Geng Q.; Auerbach A.; Li F. (2020) Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 117 (21), 11727–11734. 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X.; Chen K.; Zou J.; Han P.; Hao J.; Han Z. (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14 (2), 185–192. 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W.; Huang N.; Becavin C.; Berg M.; Queen R.; Litvinukova M.; Talavera-Lopez C.; Maatz H.; Reichart D.; Sampaziotis F.; Worlock K. B.; Yoshida M.; Barnes J. L.; (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26 (5), 681–687. 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E. J.; Limpens R.; de Wilde A. H.; de Jong A. W. M.; Zevenhoven-Dobbe J. C.; Maier H. J.; Faas F.; Koster A. J.; Barcena M. (2020) A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. PLoS Biol. 18 (6), e3000715. 10.1371/journal.pbio.3000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. R.; Lin L. D.; Machamer C. E. (2011) Identification of a Golgi complex-targeting signal in the cytoplasmic tail of the severe acute respiratory syndrome coronavirus envelope protein. J. Virol 85 (12), 5794–803. 10.1128/JVI.00060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Dellibovi-Ragheb T. A.; Kerviel A.; Pak E.; Qiu Q.; Fisher M.; Takvorian P. M.; Bleck C.; Hsu V. W.; Fehr A. R.; Perlman S.; Achar S. R.; Straus M. R.; Whittaker G. R.; de Haan C. A. M.; Kehrl J.; Altan-Bonnet G.; Altan-Bonnet N. (2020) Betacoronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 183 (6), 1520. 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis Neto E. T.; Kakehasi A. M.; de Medeiros Pinheiro M.; Ferreira G. A.; Marques C. D. L.; da Mota L. M. H.; Dos Santos Paiva E.; Pileggi G. C. S.; Sato E. I.; Reis A.; Xavier R. M.; Provenza J. R. (2020) Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv. Rheumatol 60 (1), 32. 10.1186/s42358-020-00134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N.; Zhang X.; Rosania G. R. (2011) Effect of phospholipidosis on the cellular pharmacokinetics of chloroquine. J. Pharmacol. Exp. Ther. 336 (3), 661–71. 10.1124/jpet.110.175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck J. M.; Mabotha T. E.; Ncokazi K. K.; Ambele M. A.; Taylor D.; Smith P. J.; Hoppe H. C.; Egan T. J. (2013) Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem. Biol. 8 (1), 133–7. 10.1021/cb300454t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauthe M.; Orhon I.; Rocchi C.; Zhou X.; Luhr M.; Hijlkema K. J.; Coppes R. P.; Engedal N.; Mari M.; Reggiori F. (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14 (8), 1435–1455. 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Cao R.; Zhang L.; Yang X.; Liu J.; Xu M.; Shi Z.; Hu Z.; Zhong W.; Xiao G. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30 (3), 269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrot M.; Andreani J.; Boxberger M.; Jardot P.; Fonta I.; Le Bideau M.; Duflot I.; Mosnier J.; Rolland C.; Bogreau H.; Hutter S.; La Scola B.; Pradines B. (2020) Antimalarial drugs inhibit the replication of SARS-CoV-2: an in vitro evaluation. Travel Med. Infect Dis 37, 101873. 10.1016/j.tmaid.2020.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. J.; Bergeron E.; Benjannet S.; Erickson B. R.; Rollin P. E.; Ksiazek T. G.; Seidah N. G.; Nichol S. T. (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69. 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbermintz L.; Leonardi W.; Jeong S. Y.; Sjodt M.; McComb R.; Ho C. L.; Retterer C.; Gharaibeh D.; Zamani R.; Soloveva V.; Bavari S.; Levitin A.; West J.; Bradley K. A.; Clubb R. T.; Cohen S. N.; Gupta V.; Martchenko M. (2015) Identification of agents effective against multiple toxins and viruses by host-oriented cell targeting. Sci. Rep. 5, 13476. 10.1038/srep13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian A.; Teramoto T.; Kulkarni A. A.; Bhattacharjee A. K.; Padmanabhan R. (2017) Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antiviral Res. 137, 141–150. 10.1016/j.antiviral.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Kang C. K.; Seong M. W.; Choi S. J.; Kim T. S.; Choe P. G.; Song S. H.; Kim N. J.; Park W. B.; Oh M. D. (2020) In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J. Intern. Med. 35 (4), 782–787. 10.3904/kjim.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein S. J. F.; Jacobs S.; Langendries L.; Seldeslachts L.; Ter Horst S.; Liesenborghs L.; Hens B.; Vergote V.; Heylen E.; Barthelemy K.; Maas E.; De Keyzer C.; Bervoets L.; Rymenants J.; Van Buyten T.; Zhang X.; Abdelnabi R.; Pang J.; Williams R.; Thibaut H. J.; Dallmeier K.; Boudewijns R.; Wouters J.; Augustijns P.; Verougstraete N.; Cawthorne C.; Breuer J.; Solas C.; Weynand B.; Annaert P.; Spriet I.; Vande Velde G.; Neyts J.; Rocha-Pereira J.; Delang L. (2020) Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl. Acad. Sci. U. S. A. 117 (43), 26955–26965. 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E.; Vijgen L.; Maes P.; Neyts J.; Van Ranst M. (2004) In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 323 (1), 264–8. 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J.; Coleman C. M.; Hart B. J.; Venkataraman T.; Holbrook M. R.; Kindrachuk J.; Johnson R. F.; Olinger G. G. Jr.; Jahrling P. B.; Laidlaw M.; Johansen L. M.; Lear-Rooney C. M.; Glass P. J.; Hensley L. E.; Frieman M. B. (2014) Repurposing of clinically developed drugs for treatment of Middle East Respiratory Syndrome coronavirus infection. Antimicrob. Agents Chemother. 58 (8), 4885–93. 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E.; Li S.; Vijgen L.; Rysman E.; Verbeeck J.; Van Ranst M.; Maes P. (2009) Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 53 (8), 3416–21. 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.; Pham H. T.; Xu H.; Quan Y.; Mesplede T. (2019) Antimalarial drugs and their metabolites are potent Zika virus inhibitors. J. Med. Virol. 91 (7), 1182–1190. 10.1002/jmv.25440. [DOI] [PubMed] [Google Scholar]
- Li C.; Zhu X.; Ji X.; Quanquin N.; Deng Y. Q.; Tian M.; Aliyari R.; Zuo X.; Yuan L.; Afridi S. K.; Li X. F.; Jung J. U.; Nielsen-Saines K.; Qin F. X.; Qin C. F.; Xu Z.; Cheng G. (2017) Chloroquine, a FDA-approved drug, prevents Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine 24, 189–194. 10.1016/j.ebiom.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L.; Carrasco L. (1993) Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67 (8), 4543–8. 10.1128/JVI.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T.; Nepomuceno R. R.; Nowlin D. M. (1992) Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191 (1), 387–95. 10.1016/0042-6822(92)90200-9. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R.; Thomas A.; Ranz A.; Xu C. M.; Pan H. Z. (1991) Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49 (2), 181–9. 10.1016/0166-6851(91)90062-B. [DOI] [PubMed] [Google Scholar]
- Cao R.; Hu H.; Li Y.; Wang X.; Xu M.; Liu J.; Zhang H.; Yan Y.; Zhao L.; Li W.; Zhang T.; Xiao D.; Guo X.; Li Y.; Yang J.; Hu Z.; Wang M.; Zhong W. (2020) Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 6 (9), 2524–2531. 10.1021/acsinfecdis.0c00522. [DOI] [PubMed] [Google Scholar]
- Gendrot M.; Duflot I.; Boxberger M.; Delandre O.; Jardot P.; Le Bideau M.; Andreani J.; Fonta I.; Mosnier J.; Rolland C.; Hutter S.; La Scola B.; Pradines B. (2020) Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: in vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int. J. Infect. Dis. 99, 437–440. 10.1016/j.ijid.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.; Yuan M.; Li H.; Deng C.; Wang Q.; Tang Y.; Zhang H.; Yu W.; Xu Q.; Zou Y.; Yuan Y.; Guo J.; Jin C.; Guan X.; Xie F.; Song J. (2020) Safety and efficacy of artemisinin-piperaquine for treatment of COVID-19: an open-label, non-randomised and controlled trial. Int. J. Antimicrob. Agents 106216. 10.1016/j.ijantimicag.2020.106216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P.; Lagier J. C.; Parola P.; Hoang V. T.; Meddeb L.; Mailhe M.; Doudier B.; Courjon J.; Giordanengo V.; Vieira V. E.; Tissot Dupont H.; Honore S.; Colson P.; Chabriere E.; La Scola B.; Rolain J. M.; Brouqui P.; Raoult D. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56 (1), 105949. 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendaal F. R. (2020) Review of: ″Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al 2010. Int. J. Antimicrob. Agents 56 (1), 106063. 10.1016/j.ijantimicag.2020.106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels J. D.; Bleeker-Rovers C. P.; Ter Heine R.; Rahamat-Langendoen J.; de Mast Q.; Ten Oever J.; Bousema T.; van Crevel R.; Wertheim H. F. (2020) Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks?. Int. J. Antimicrob. Agents 56 (1), 106056. 10.1016/j.ijantimicag.2020.106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J. M.; Delaugerre C.; Le Goff J.; Mela-Lima B.; Ponscarme D.; Goldwirt L.; de Castro N. (2020) No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal Infect 50 (4), 384. 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad S.; Kilgore P.; Chaudhry Z. S.; Jacobsen G.; Wang D. D.; Huitsing K.; Brar I.; Alangaden G. J.; Ramesh M. S.; McKinnon J. E.; O’Neill W.; Zervos M.; (2020) Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 97, 396–403. 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A. C. S.; Antinori A.; Berselli N.; Blandi L.; Bruno R.; Cauda R.; Guaraldi G.; Menicanti L.; My I.; Parruti G.; Patti G.; Perlini S.; Santilli F.; Signorelli C.; Spinoni E.; Stefanini G. G.; Vergori A.; Ageno W.; Agodi A.; Aiello L.; Agostoni P.; Al Moghazi S.; Astuto M.; Aucella F.; Barbieri G.; Bartoloni A.; Bonaccio M.; Bonfanti P.; Cacciatore F.; Caiano L.; Cannata F.; Carrozzi L.; Cascio A.; Ciccullo A.; Cingolani A.; Cipollone F.; Colomba C.; Crosta F.; Dal Pra C.; Danzi G. B.; D’Ardes D.; de Gaetano Donati K.; Del Giacomo P.; Di Gennaro F.; Di Tano G.; D’Offizi G.; Filippini T.; Fusco F. M.; Gentile I.; Gialluisi A.; Gini G.; Grandone E.; Grisafi L.; Guarnieri G.; Lamonica S.; Landi F.; Leone A.; Maccagni G.; Maccarella S.; Madaro A.; Mapelli M.; Maragna R.; Marra L.; Maresca G.; Marotta C.; Mastroianni F.; Mazzitelli M.; Mengozzi A.; Menichetti F.; Meschiari M.; Minutolo F.; Montineri A.; Mussinelli R.; Mussini C.; Musso M.; Odone A.; Oliveri M.; Pasi E.; Petri F.; Pinchera B.; Pivato C. A.; Poletti V.; Ravaglia C.; Rinaldi M.; Rognoni A.; Rossato M.; Rossi I.; Rossi M.; Sabena A.; Salinaro F.; Sangiovanni V.; Sanrocco C.; Scorzolini L.; Sgariglia R.; Simeone P. G.; Spinicci M.; Trecarichi E. M.; Venezia A.; Veronesi G.; Vettor R.; Vianello A.; Vinceti M.; Vocciante L.; De Caterina R.; Iacoviello L. (2020) Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: findings from the observational multicentre Italian CORIST study. Eur. J. Intern Med. 82, 38–47. 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catteau L.; Dauby N.; Montourcy M.; Bottieau E.; Hautekiet J.; Goetghebeur E.; van Ierssel S.; Duysburgh E.; Van Oyen H.; Wyndham-Thomas C.; Van Beckhoven D.; et al. (2020) Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants. Int. J. Antimicrob. Agents 56 (4), 106144. 10.1016/j.ijantimicag.2020.106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleris J.; Sun Y.; Platt J.; Zucker J.; Baldwin M.; Hripcsak G.; Labella A.; Manson D. K.; Kubin C.; Barr R. G.; Sobieszczyk M. E.; Schluger N. W. (2020) Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 382 (25), 2411–2418. 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E. S.; Dufort E. M.; Udo T.; Wilberschied L. A.; Kumar J.; Tesoriero J.; Weinberg P.; Kirkwood J.; Muse A.; DeHovitz J.; Blog D. S.; Hutton B.; Holtgrave D. R.; Zucker H. A. (2020) Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 323 (24), 2493–2502. 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip A.; Berry D. A.; Hansen E.; Goy A. H.; Pecora A. L.; Sinclaire B. A.; Bednarz U.; Marafelias M.; Berry S. M.; Berry N. S.; Mathura S.; Sawczuk I. S.; Biran N.; Go R. C.; Sperber S.; Piwoz J. A.; Balani B.; Cicogna C.; Sebti R.; Zuckerman J.; Rose K. M.; Tank L.; Jacobs L. G.; Korcak J.; Timmapuri S. L.; Underwood J. P.; Sugalski G.; Barsky C.; Varga D. W.; Asif A.; Landolfi J. C.; Goldberg S. L. (2020) Hydroxychloroquine and tocilizumab therapy in COVID-19 patients - an observational study. PLoS One 15 (8), e0237693. 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahevas M.; Tran V. T.; Roumier M.; Chabrol A.; Paule R.; Guillaud C.; Fois E.; Lepeule R.; Szwebel T. A.; Lescure F. X.; Schlemmer F.; Matignon M.; Khellaf M.; Crickx E.; Terrier B.; Morbieu C.; Legendre P.; Dang J.; Schoindre Y.; Pawlotsky J. M.; Michel M.; Perrodeau E.; Carlier N.; Roche N.; de Lastours V.; Ourghanlian C.; Kerneis S.; Menager P.; Mouthon L.; Audureau E.; Ravaud P.; Godeau B.; Gallien S.; Costedoat-Chalumeau N. (2020) Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 369, m1844. 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry C. A.; Humphrey M. B.; Thind S. K.; Hendrickson S. C.; Kurdgelashvili G.; Williams R. J. 2nd (2020) Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study. Lancet Rheumatol 2 (11), e689–e697. 10.1016/S2665-9913(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.; Tang T.; Pang P.; Li M.; Ma R.; Lu J.; Shu J.; You Y.; Chen B.; Liang J.; Hong Z.; Chen H.; Kong L.; Qin D.; Pei D.; Xia J.; Jiang S.; Shan H. (2020) Treating COVID-19 with chloroquine. J. Mol. Cell Biol. 12 (4), 322–325. 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti A. B.; Zampieri F. G.; Rosa R. G.; Azevedo L. C. P.; Veiga V. C.; Avezum A.; Damiani L. P.; Marcadenti A.; Kawano-Dourado L.; Lisboa T.; Junqueira D. L. M.; de Barros E. S. P. G. M.; Tramujas L.; Abreu-Silva E. O.; Laranjeira L. N.; Soares A. T.; Echenique L. S.; Pereira A. J.; Freitas F. G. R.; Gebara O. C. E.; Dantas V. C. S.; Furtado R. H. M.; Milan E. P.; Golin N. A.; Cardoso F. F.; Maia I. S.; Hoffmann Filho C. R.; Kormann A. P. M.; Amazonas R. B.; Bocchi de Oliveira M. F.; Serpa-Neto A.; Falavigna M.; Lopes R. D.; Machado F. R.; Berwanger O.; (2020) Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 383 (21), 2041–2052. 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P.; Mafham M.; Linsell L.; Bell J. L.; Staplin N.; Emberson J. R.; Wiselka M.; Ustianowski A.; Elmahi E.; Prudon B.; Whitehouse T.; Felton T.; Williams J.; Faccenda J.; Underwood J.; Baillie J. K.; Chappell L. C.; Faust S. N.; Jaki T.; Jeffery K.; Lim W. S.; Montgomery A.; Rowan K.; Tarning J.; Watson J. A.; White N. J.; Juszczak E.; Haynes R.; Landray M. J. (2020) Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 383 (21), 2030–2040. 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.; Peto R.; Henao-Restrepo A. M.; Preziosi M. P.; Sathiyamoorthy V.; Abdool Karim Q.; Alejandria M. M.; Hernandez Garcia C.; Kieny M. P.; Malekzadeh R.; Murthy S.; Reddy K. S.; Roses Periago M.; Abi Hanna P.; Ader F.; Al-Bader A. M.; Alhasawi A.; Allum E.; Alotaibi A.; Alvarez-Moreno C. A.; Appadoo S.; Asiri A.; Aukrust P.; Barratt-Due A.; Bellani S.; Branca M.; Cappel-Porter H. B. C.; Cerrato N.; Chow T. S.; Como N.; Eustace J.; Garcia P. J.; Godbole S.; Gotuzzo E.; Griskevicius L.; Hamra R.; Hassan M.; Hassany M.; Hutton D.; Irmansyah I.; Jancoriene L.; Kirwan J.; Kumar S.; Lennon P.; Lopardo G.; Lydon P.; Magrini N.; Maguire T.; Manevska S.; Manuel O.; McGinty S.; Medina M. T.; Mesa Rubio M. L.; Miranda-Montoya M. C.; Nel J.; Nunes E. P.; Perola M.; Portoles A.; Rasmin M. R.; Raza A.; Rees H.; Reges P. P. S.; Rogers C. A.; Salami K.; Salvadori M. I.; Sinani N.; Sterne J. A. C.; Stevanovikj M.; Tacconelli E.; Tikkinen K. A. O.; Trelle S.; Zaid H.; Rottingen J. A.; Swaminathan S. (2021) Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. 384 (6), 497–511. 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abella B. S.; Jolkovsky E. L.; Biney B. T.; Uspal J. E.; Hyman M. C.; Frank I.; Hensley S. E.; Gill S.; Vogl D. T.; Maillard I.; Babushok D. V.; Huang A. C.; Nasta S. D.; Walsh J. C.; Wiletyo E. P.; Gimotty P. A.; Milone M. C.; Amaravadi R. K.; (2021) Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 181 (2), 195–202. 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D. R.; Pullen M. F.; Bangdiwala A. S.; Pastick K. A.; Lofgren S. M.; Okafor E. C.; Skipper C. P.; Nascene A. A.; Nicol M. R.; Abassi M.; Engen N. W.; Cheng M. P.; LaBar D.; Lother S. A.; MacKenzie L. J.; Drobot G.; Marten N.; Zarychanski R.; Kelly L. E.; Schwartz I. S.; McDonald E. G.; Rajasingham R.; Lee T. C.; Hullsiek K. H. (2020) A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 383 (6), 517–525. 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University . COVID-19 dashboard for the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). See the following: https://coronavirus.jhu.edu/map.html. [Google Scholar]
- Qiu T.; Liang S.; Dabbous M.; Wang Y.; Han R.; Toumi M. (2020) Chinese guidelines related to novel coronavirus pneumonia. J. Mark Access Health Policy 8 (1), 1818446. 10.1080/20016689.2020.1818446. [DOI] [PMC free article] [PubMed] [Google Scholar]