Abstract
The limited impact of treatments for COVID-19 has stimulated several Phase 1 clinical trials of whole-lung low-dose radiation therapy (LDRT; 0.3 – 1.5 Gy) that are now proceeding to Phase 2 randomized trials worldwide. This novel but unconventional use of radiation to treat COVID-19 prompted the National Cancer Institute, National Council on Radiation Protection and Measurements, and National Institute of Allergy and Infectious Diseases to convene a workshop involving a diverse group of experts in radiation oncology, radiobiology, virology, immunology, radiation protection, and public health policy. The workshop discussed the mechanistic underpinnings, rationale, and preclinical and emerging clinical studies, and developed a general framework for use in clinical studies. Without refuting or endorsing LDRT as a treatment for COVID-19, the purpose of the workshop and this review is to provide guidance to clinicians and researchers who plan to conduct preclinical and clinical studies, given the limited available evidence on its safety and efficacy.
Introduction
To improve clinical outcomes for COVID-19 patients, a variety of experimental treatments are being evaluated, including clinical trials for whole-lung low-dose radiation therapy (LDRT) (1, 2), defined here as 0.3 to 1.5 Gy delivered in a single fraction, which is lower than doses used in clinical radiotherapy and higher than the 50 mSv/year occupational exposure limit and 1 mSv/year limit to the general public (https://ncrponline.org/shop/reports/report-no-180-management-of-exposure-to-ionizing-radiation-radiation-protection-guidance-for-the-united-states-2018-2018/).
Clinical trials currently testing whole-lung, LDRT for COVID-19 are listed in Table 1. Most of these trials are open-label, single institution, non-randomized studies, but some randomized trials are also emerging. Among several hospital centers worldwide, enrollment of 982 patients is planned to date. To address this unconventional treatment’s uniqueness and accompanying scientific controversy (3, 4), the National Cancer Institute (NCI), National Council on Radiation Protection and Measurements (NCRP), and National Institute of Allergy and Infectious Diseases (NIAID) convened a virtual workshop on July 23, 2020 to discuss available data concerning potential risks/benefits of LDRT. One main goal of the workshop was to develop guidelines for future clinical trials by developing components for inclusion in clinical studies, taking into consideration pros and cons of several parameters that would address the pathogenesis and underlying mechanisms of COVID-19.
Table 1.
Completed and Ongoing Clinical Trials on Whole-Lung LDRT for COVID-19 Registered in the ClinicalTrials.gov
| Title of the Trial | Status | Conditions | Locations | Interventions | Estimated Enrollment | ClinicalTrials.gov Id |
|---|---|---|---|---|---|---|
| Low Dose Whole Lung Radiotherapy for Older Patients With COVID-19 Pneumonitis | NYR | Covid-19 Pneumonitis | International Geriatric Radiotherapy Group, Institute of Radiation Oncology, Cantonal Hospital Graubuenden, Chur, Switzerland | Radiation: Whole lung LDRT for older patients with COVID-19 pneumonitis | 500 | NCT04493294 |
| Radiation Eliminates Storming Cytokines and Unchecked Edema as 1-Day Treatment for COVID-19 (RESCUE 1–19) | R | Pneumonia| COVID19 |SARS Pneumonia | Emory University Hospital, Winship Cancer Institute, Atlanta, GA, USA | Radiation: LDRT | 10 | NCT04366791 |
| COVID-19 Pneumonitis Low Dose Lung RT (COLOR-19) | R | COVID-19 | ASST SpedaliCivili, Brescia, Italy | Radiation: Single fraction whole lung RT | 30 | NCT04377477 |
| Low Dose Anti-inflammatory RT for the Treatment of Pneumonia by COVID-19 | R | Pneumonia, Viral | Hospital Sant Joan de Reus, Reus, Tarragona, Spain | Radiation: LDRT| Drug: Hydroxychloroquine Sulfate| Drug: Ritonavir/lopinavir| Drug: Tocilizumab Injection [Actemra]| Drug: Azithromycin| Drug: Corticosteroid| Drug: Low molecular weight heparin| Device: Oxygen supply | 106 | NCT04380818 |
| LDRT in COVID-19 Pneumonia | R | COVID| SARS | Imam Hossein Hospital, Tehran, Iran | Radiation: LDRT | 5 | NCT04390412 |
| Lung Irradiation for COVID-19 Pneumonia | R | SARS-CoV 2 | Brigham and Women’s Hospital, Boston, MA, USA | Radiation: Phase 1| Radiation: Phase 2 | 48 | NCT04393948 |
| Ultra-Low Doses of Therapy with Radiation Applicated to COVID-19 | R | Pneumonia, Viral| Cytokine Storm | Hospital La Milagrosa, GenesisCare, Madrid, Spain | Radiation: Ultra-Low-dose RT| Device: ventilatory support with oxygen therapy| Drug: Lopinavir/ritonavir| Drug: Hydroxychloroquine| Drug: Azithromycin| Drug: Piperacillin/tazobactam| Drug: Low molecular weight heparin| Drug: Corticosteroid injection| Drug: Tocilizumab | 15 | NCT04394182 |
| LDRT for COVID-19 Pneumonia: A Pilot Study | R | COVID-19| Pneumonia | All India Institute of Medical Sciences, New Delhi, India | Radiation: LDRT | 10 | NCT04394793 |
| Low Dose Pulmonary Irradiation in Patients With COVID-19 Infection of Bad Prognosis | NYR | COVID| Pneumonia, Viral | Hospital Provincial de Castellon, Castellón De La Plana, Castellon, Spain | Radiation: Lung LDRT | 41 | NCT04414293 |
| LDRT for COVID-19 Pneumonitis | R | COVID-19 | Servicio de Oncología Radioterápica. Hospital Clínico San Carlos, Madrid, Spain | Radiation: RT | 41 | NCT04420390 |
| Low Dose Whole Lung RT for Patients With COVID-19 and Respiratory Compromise | R | COVID-19 | Arthur G. James Cancer Hospital and Solove Research Institute at Ohio State University Medical Center, Columbus, Ohio, USA | Radiation: RT | 24 | NCT04427566 |
| Best Supportive Care with or without Low Dose Whole Lung RT for the Treatment of COVID-19 | R | Pneumonia| Severe Acute Respiratory Syndrome| Symptomatic COVID-19 Infection Laboratory-Confirmed | Emory University Hospital, Winship Cancer Institute, Atlanta, GA, USA | Other: Best Practice| Radiation: LDRT | 52 | NCT04433949 |
| LDRT For Patients With SARS-COV-2 (COVID-19) Pneumonia | NYR | Covid-19| Sars-CoV2| Pneumonia | Ohio State University Medical Center, Columbus, Ohio, USA | Radiation: Low dose radiation 35 cGy| Radiation: High dose radiation 100 cGy | 100 | NCT04466683 |
Abbreviations: R, recruiting; NYR, not yet recruiting
The workshop brought together a diverse group of scientific experts in radiation oncology, pulmonary virology, immunology, vascular- and radiation-biology, radiation protection, and public health policy. There were 3 sessions: clinical trials/trial design, preclinical studies, and radiobiological/ immunological mechanisms of LDRT. The available results of clinical trials conducted to date, including those just accruing patients and those being implemented and in the final planning phase, served as a key focal point of debate about the need for preclinical data prior to initiating trials and the protocol design and methodology. Issues discussed included those related to COVID-19 biology, low-dose radiation biology, radiation lung injury and how these complex mechanisms might interact. Given the use of ionizing radiation for a purpose other than treating cancer, potential life-time radiation risks and regulatory aspects of the use of radiation for LDRT were discussed. Without supporting or refuting LDRT for COVID-19 as a treatment, this review is to provide guidance to clinicians and researchers who plan to conduct preclinical and clinical studies, given the limited evidence on its safety and efficacy. Also, we recognize the need for novel treatments for patients in a dire clinical condition, however, it is essential that any use of LDRT for COVID-19 be on a clinical trial with Institutional Review Board (IRB) oversight, and investigators must provide complete details of trials including patient selection, tracking those who were eligible, consented, received LDRT, and report both short- and long-term results.
Clinical trials and trial design
The workshop participants recognized the dire need for effective treatments as well as scientific consensus from the NCRP (https://ncrponline.org/shop/reports/report-no-186-approaches-for-integrating-information-from-radiation-biology-and-epidemiology-to-enhance-low-dose-health-risk-assessment-2020/), the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR; https://www.unscear.org/unscear/en/publications/2006_1.html), and others that radiation doses > 0.1 Gy carry long-term risks, which include cancer and cardiovascular events, modulated by several factors such as age at time of exposure and sex (3, 5, 6).
The impetus for LDRT comes from case studies of patients treated with thoracic radiation for pneumonia between 1905 and 1943, which suggest clinical improvement (7, 8). As the studies lacked a randomized control arm without radiation exposure, it is difficult to establish whether treatment impacted the course of the disease. Given the paucity of preclinical data and limited interpretability of the historical data, some workshop participants insisted on the need to establish the efficacy of LDRT in animal models of SARS-CoV-2 before testing in human clinical trials, opining that LDRT is not indicated (9). Others argued for continuation of IRB-approved clinical trials based on the need to investigate treatments that aim to reduce COVID-19 morbidity and mortality and on initial Phase I trial observations. While several other COVID-19 therapies are being evaluated, to date, only two drugs have shown improved outcomes: the steroid Dexamethasone lowers the odds of death (10), and the antiviral Remdesivir shortens oxygen dependency and recovery time but does not improve survival (11). Ultimately, management of this pandemic will be modified by improved understanding of pathogenesis of tissue injury, immunological/inflammatory response, the availability of preventive and therapeutic vaccines, and continually updated clinical guidance (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html).
The risk/benefit considerations for LDRT include the possibility of short-term worsening of the disease course (to be determined by Phase 1 and 2 clinical trials) and long-term population-based radiation risks should a large number of patients live many years/decades vs. the potential benefit of reduced mortality and morbidity in the most severely ill patients in the immediate term (days/weeks). While speculative, it is conceivable that whole-lung LDRT could exacerbate SARS-CoV-2 infection. Autopsies of patients who died from COVID-19 show infection of endothelial cells with associated endothelial inflammation, vasculopathy, and microangiopathy (12, 13). In general, exposure to doses >2 Gy are likely to induce endothelial activation (14). Endothelial molecules, ICAM-1 (15) and E-selectin (16), are upregulated in a dose- and time-dependent manner, in part due to NF-κB activation (17), in preclinical studies. Because the endothelial cells in small vessel capillaries in the lung can potentially be damaged by ionizing radiation (14), it is conceivable that LDRT might promote endothelial cell injury and coagulopathy in the COVID lungs due to combined injury. Further, there might be differences in the dynamics of response of endothelial cells between preclinical models and humans to a given dose of radiation. Therefore, characterization of dose-effect and time-course responses of endothelial cells to radiation are essential to fully understand the adverse effects of LDRT.
The general rationale for LDRT is that it could inhibit the cytokine storm that promotes the virus-induced pulmonary dysfunction contributing to the development of Acute Respiratory Distress Syndrome (ARDS). It is also likely that the potential anti-viral effect of LDRT could be due to the activation of immune and endothelial cells and inhibition of subsequent viral loading. However, preclinical data are necessary to support or refute this notion. LDRT as a treatment for COVID-19 patients to prevent respiratory failure, based on its role in treating inflammatory and degenerative diseases, is reviewed (18). Use of LDRT requires meticulous planning to overcome the challenges, including: (i) determining the optimum time, if any, during disease progression to treat COVID-19 with LDRT, considering its safety and efficacy, (ii) safety of healthcare personnel during patient transport to the RT facility, (iii) rapid deterioration of eligible and enrolled patient’s medical condition, and (iv) availability of RT facilities only on certain days (in many centers), potentially causing a delay between obtaining informed consent and the actual treatment delivery. Patients who deteriorate during this delay and fail to receive LDRT influence the interpretation of the existing preliminary Phase 1 data.
The first published peer-reviewed results from the U.S. are from a safety trial of 1.5 Gy LDRT in elderly patients (median age 90), with bilateral pulmonary infiltrates, oxygen dependence without mechanical ventilation, and high comorbidity burdens ‒ factors known to predispose patients to a worse outcome (2). A pre-specified interim safety analysis on day 7 was the primary endpoint: 7 patients were enrolled (but 1 was transferred to the ICU and 1 expired prior to LDRT); thus, 5 received radiation. No acute worsening of the cytokine storm nor acute events were noted in 4 patients whose clinical status improved over 3 to 96 hours post LDRT. The trial met the primary safety endpoint with day 7 survival in the 5 irradiated patients.
In Iran, 5 patients with COVID-19 on supplemental oxygen were treated with 0.5 Gy whole lung RT: 1 died, 1 withdrew consent, and 3 showed clinical improvement so that they could be discharged, with one patient discharged with oxygen support at home (1). As these patients were not treated with Dexamethasone, the death of 1 of 4 evaluable patients from COVID-19 is consistent with the expected mortality of 25% for hospitalized patients on supplemental oxygen (19). The authors reported a response rate of 4 out of 5 initially enrolled patients, based on an initial improvement in oxygen saturation and body temperature within 1 day after LDRT. Collectively, these studies established the feasibility of 0.5 to 1.5 Gy whole lung irradiation in COVID-19 patients on supplemental oxygen.
Two trials underway that were discussed include i) a Phase 2, single-arm study of 30-day mortality for patients requiring mechanical ventilation (NCT04427566) to receive 0. 8 Gy LDRT and possibly other investigational treatments; and ii) a randomized, multi-institutional study for patients requiring hospitalization for hypoxemia, fever, or pulmonary compromise, but not mechanical ventilation (NCT04466683). In addition to experimental drugs, initial randomization is to LDRT either 0.35 Gy or 1 Gy; following interim pre-specified evaluation, randomization will be to standard of care (SOC) or SOC plus the more efficacious/safer LDRT dose.
Preclinical translational approaches
The Food and Drug Administration (FDA) informed that RT devices are cleared for certain indications such as treating tumors in adults and children, but their use in treating COVID-19 or modulating the inflammatory response in patients with COVID-19 are not cleared indications. Other regulatory pathways available include Emergency Use Authorization (EUA) (https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization) and Investigational Device Exemptions (IDEs) (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=812&showFR=1). EUAs are in effect only during a public health emergency; no RT devices currently have an EUA for COVID-19. A physician may choose to use RT to treat a patient for COVID-19 associated pathologies as part of the practice of medicine; however, treating clinicians should be well-informed about the treatment and base their decision on solid scientific and medical evidence. IRB oversight and informed consent may be required by the physician’s institution before treatment. The practice of medicine does not include use of a device in clinical trials.
The importance of considering correlative, preclinical work in the design and conduct of LDRT clinical studies was discussed using a schema of the clinical and pathological evolution of COVID-19 infection. Figure 1 shows the chronology of events that leads to the cytokine storm in SARS-CoV-2 infection, causing a dysfunctional immune response and ultimately ARDS (20).
FIG. 1.
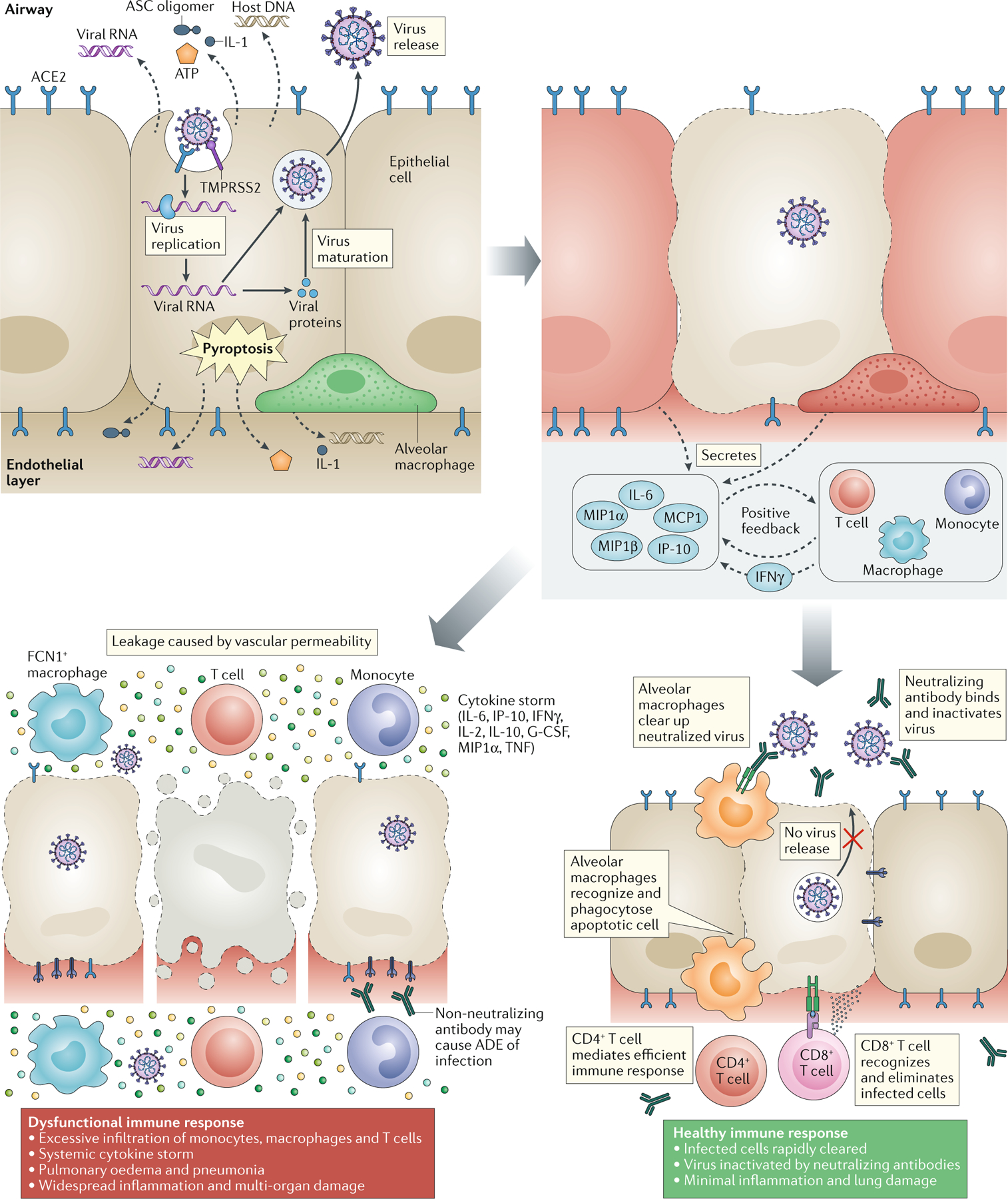
Chronology of Events Leading to the Cytokine Storm in SARS-CoV-2 Infection Causing Dysfunctional Immune Response and Ultimately ARDS (modified with permission)(20).
The pathological evolution toward severe ailment does not always depend on viral load and culminates in a combination of symptoms. These include lymphopenia, with a relative abundance of abnormally functioning or exhausted CD8+ T cells, high neutrophil-to-lymphocyte ratio, increased antibody-secreting B cells, and monocytopenia with a relative increase in inflammatory CD14+ and CD16+ monocytes. Also observed are increases in multiple cytokines (e.g., IL-2, IL-6, IL-7, IL-10) and chemokines responsible for recruiting neutrophils to the lung. The evidence for some effectiveness of anti-viral therapy during the moderate phase and steroid therapy during the severe phase was also presented. Based on the discussions, suggested correlative studies for clinical trials, are provided in Table 2.
Table 2:
| • Peripheral blood mononuclear cells (PBMC) – flow cytometry, CyTOF® |
| • Plasma – cytokines, miRNA, exosomes, proteins/metabolites, neutralizing antibodies, hypoxia markers |
| ○ Radiation pneumonitis markers (TGF-β, KL-6, surfactants, IL-1ra, IL-6, sTNF, C3, C4b-binding protein-α) |
| ○ Endothelial/cardiac injury markers (hscTnT, CKMB, NT-proBNP, ANP) |
| ○ Systemic inflammation markers (CRP, LDH, ferritin, D-dimer, IL-6) |
| • Bronchial secretions (BAL fluid) – viral load, flow cytometry |
| • Pulmonary function tests, oxygen requirements, ventilator settings, radiographic imaging, dosimetry |
| • Stool – viral load |
| • Co-morbidities – concurrent medications, prior treatments, quality-of-life |
A reanalysis of several animal datasets from experiments performed decades ago testing RT for bacterial and viral pneumonia reveal that results are heterogeneous, but collectively indicate lack of efficacy of RT after infection (https://arxiv.org/abs/2008.02625v3). Animal models of SARS-CoV-2 infection (while not identical to human COVID-19) include non-human primates, hamsters, and transgenic mice expressing the human ACE2 receptor. However, no studies of LDRT in these models are available (3), and some workshop participants called for such pre-clinical experiments before continued testing of LDRT in COVID-19 patients.
Radiobiological and immunological mechanisms
Using X-rays to treat non-malignant disorders, including pneumonia (23) was more common in the U.S. until the 1950s, although LDRT is used in Germany, mainly for degenerative, hyperproliferative or inflammatory diseases (24), where single doses of 0.5 – 1.0 Gy and total doses of 3.0 – 6.0 Gy are delivered to patients > 40 yr.(24) Rapid decreases in pain and edema are commonly reported. The relevance to LDRT for COVID-19 can be questioned; however, efficacy suggests an anti-inflammatory action, supported by preclinical studies (25).
Our understanding of the biology of SARS-CoV-2 is constantly evolving and studies on the effects of radiation on COVID-19 are limited and challenging. This complex biology adds to the debate about the essential need for preclinical data and clinical trials, to include carefully specified indications, exclusions, treatment regimens, biomarkers and clinical outcomes, and the importance of a randomized study comparing radiation vs. drug therapy or drug therapy +/− LDRT. Selecting radiation dose may be from a randomization (noted in the study above) or by the investigators’ choice.
SARS-CoV-2 in the lung targets primarily bronchial and alveolar epithelial cells, but other targets are likely, including immune cells. Pathogen-associated molecular patterns are sensed by innate pattern recognition receptors (PRR) on resident immune and non-immune cells, leading to the production of chemokines and cytokines. These molecules activate leukocyte-endothelial interactions to allow an influx of immune cells into the inflammatory site. The PRRs that sense the presence of SARS-CoV-2 have yet to be fully identified, but are likely important for the outcome of infection and could explain why this virus stimulates a vigorous hyperinflammatory response (26). Irradiation is known to modulate toll-like receptor (TLR) expression, which could alter early innate immune response (27), although it is unknown whether LDRT can do this. Immune cells, especially the myeloid subsets, are activated to phagocytose debris in the inflammatory site while dendritic cells (DC) present antigen to stimulate adaptive immunity in primary lymphoid tissues. Myeloid cells, however, appear to be dysregulated (see later) and conventional DCs and pDCs are decreased in COVID-19 patients (28), but whether the generation of anti-viral immunity is affected is, as yet, unclear.
Inflammation is a multistep process involving many cell types and mediators such as reactive oxygen species (ROS), nitric oxide, prostaglandins, and pro-inflammatory chemokines and cytokines especially interleukin (IL)-1, tumor necrosis factor-α (TNF-α), IL-6, IL-8, and IL-12. Pro-inflammatory cytokines also tie into coagulation pathways and fibrin deposition, which is important in the later stages of COVID-19 pathogenesis. Vascular endothelial cells play a central role in responding to and producing cytokines that further drive cytokine production and lead to a coagulation response, mainly through the extrinsic pathway involving tissue factor and Factor VIIa. Such inflammatory, pro-coagulation events can overwhelm host defenses, leading to disseminated intravascular coagulation and multiple organ failure. Coagulopathy is frequently associated with excessive systemic inflammation and is a distinct feature of advanced COVID-19 (29). The ability of single high doses of radiation to induce inflammation and trigger clotting has been known for decades (30). In contrast, little is known as to how LDRT might affect the coagulation process, particularly in ARDS. The effects of LDRT on ongoing inflammatory processes have been investigated in other model systems. However, detailed dose-response and time-course studies are essential to deciphering the pro-survival immune-modulatory response vs. cytotoxic effects of LDRT on lung vascular endothelial cells. Since endothelial cells are relatively radiosensitive, LDRT-mediated benefit may be linked to an inhibitory effect on coagulopathy, which needs to be verified.
The effects of LDRT on many inflammatory processes have been investigated, but data are not consistent with respect to radiation dose and time of response. A general picture however has emerged of anti-inflammatory actions of doses in the range 0.1 – 1.0 Gy with notably reduced immune cell/endothelial cell adhesion and production of inflammatory mediators such as reactive oxygen species, nitric oxide, and cytokines in vitro that translated in vivo to LDRT efficacy on experimental collagen-induced arthritis (31, 32), experimental autoimmune encephalomyelitis (33), TNF-induced polyarthritis (34), and decreased neuroinflammation in mouse models of Alzheimer’s disease (35, 36). Although these LDRT studies were performed in the context of inflammatory conditions, it should be noted that LDRT was often delivered just before or after a challenge. There is a paucity of data on the effects of LDRT in preclinical animal models in the context of viral infection. Many factors, including IL-6 (37), affect radiation responses, and the use of an appropriate animal model is crucial as a preexisting inflammation can dramatically alter the response to radiation, especially if it is hyperinflammatory.
Pathophysiological changes in vessel-lung interface due to SARS-CoV-2 infection leading to the loss of vascular integrity, activation of the coagulation pathway, and inflammation have been recently published (38) (Fig. 2). In its worst form, COVID-19 is a complex pathophysiological disease state, which includes severe pneumonia, ARDS, septic shock syndrome, disseminated intravascular coagulation, and multiorgan failure (39, 40). In patients, IL-2, IL-4, IL-6, IL-7, IL-9, IL-10, G-CSF, IP10/CXCL10, MCP1/CCL2, MIP1A, CCL3, and TNF-alpha are frequently elevated.(41) High levels of a subset of these pro-inflammatory chemokines and cytokines, especially IL-6, correlate directly with disease severity and could be responsible for at least some of the disease’s pathological features (42). Given these findings, it is not surprising that attempts are being made to target inflammation in COVID-19 (43), including IL-6 blocking antibodies, IL-1R agonists, NADPH oxidase (NOX) inhibitors, dexamethasone, JAK1 inhibitors, and now LDRT.
FIG. 2.
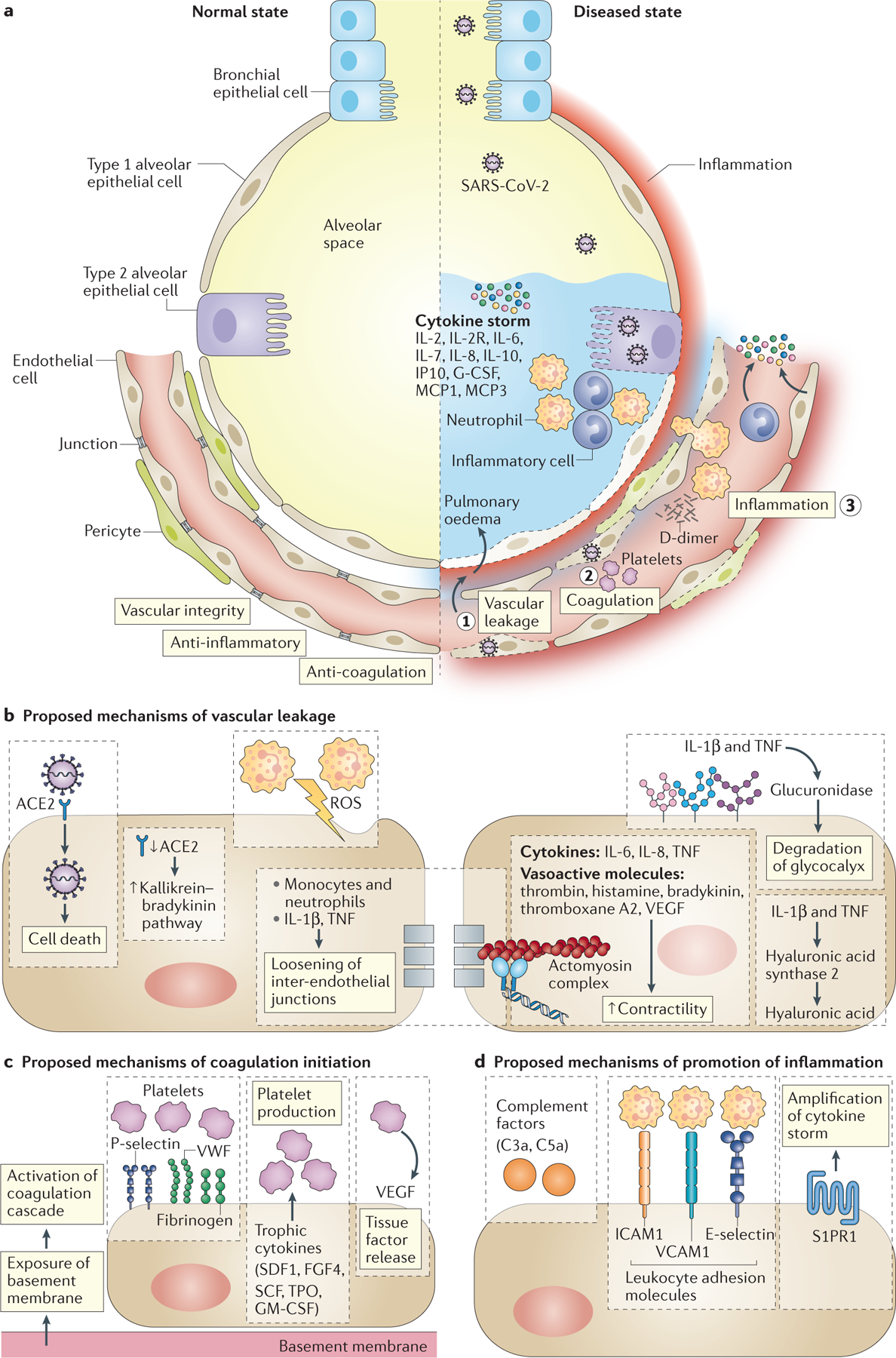
Pathophysiological changes in vessel-lung interface due to SARS-CoV-2 infection leading to the loss of vascular integrity, activation of the coagulation pathway, and inflammation (modified with permission)(38).
Figure 3 illustrates the timeline of dynamic changes in lungs resulting from SARS-CoV-2 exposure, causing anti-viral immune response and inflammatory response, leading to ARDS and multiorgan failure due to hyperinflammation. This mechanistic understanding of pathophysiological processes resulting from early infection and pulmonary phase to the development of ARDS due to hyperinflammation, provides a “hypothetical window of opportunity” to intervene in the disease progression with LDRT; however, this concept needs to be tested and confirmed with biomarkers of immune and inflammatory responses (see Tables 2 and 3) alongside tissue oxygenation status (Fig. 3).
FIG. 3:
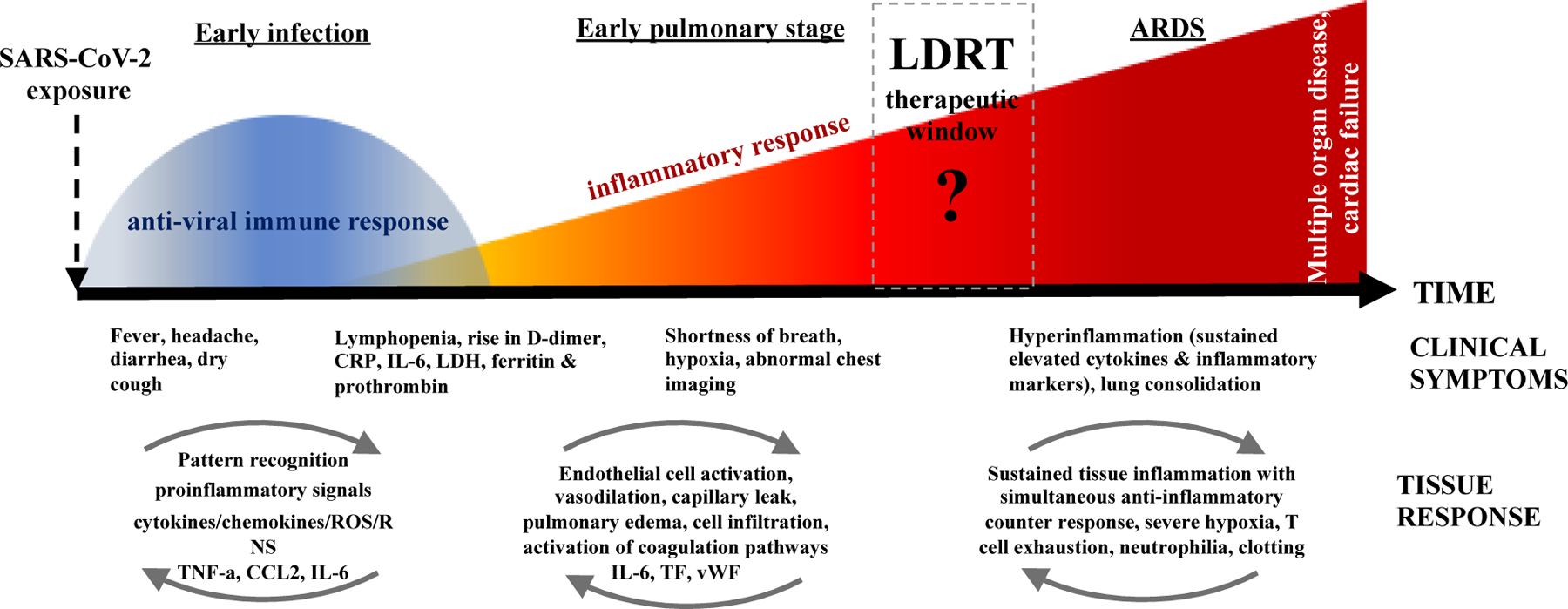
Timeline of the dynamic changes resulting from SARS-CoV-2 exposure. The current understanding of the pathophysiological processes from the early anti-viral immune response to the development of ARDS and multi-organ failure due to hyperinflammation, opens a “hypothetical window of opportunity” for intervention with LDRT. However, this is a concept that needs to be tested and confirmed with biomarkers of immune and inflammatory response (see Tables and 3 for potential biomarkers) alongside tissue oxygenation status. The LDRT window of opportunity will likely follow the principle of ‘as early as possible and as late as necessary’ but the suggestion is that LDRT will be most effective during the early stages of inflammation, with the caveat of potential adverse effects of lung radiation exposure and combined injury.
Table 3.
Suggested Parameters for the Study Protocol, if Low Dose Radiation Therapy (LDRT) is Planned to be Tested for COVID-19
| Section | Protocol Content/Stipulation | Comments/Concerns to Address | |
|---|---|---|---|
| Background | Rationale for the use of LDRT for COVID-19 | Biology of disease and radiation Specific biological rationale for use and timing of LDRT |
|
| Objectives of LDRT | |||
| Primary | Presumably anti-inflammatory or this can be immunomodulatory (therapeutic effect of vaccine) | Address anti-inflammatory effect(s) | |
| Secondary | Development of biomarker panels in the context of LDRT use, to aid in acute clinical management and in the analysis of late clinical trajectories of COVID-19 (55–66) | Address any anti-viral effect(s) as well as potential immune enhancement of disease Address coagulopathy effect(s) |
|
| Patient Selection | |||
| Eligibility | Clinical condition and measurable parameters; Specify clinical criteria in terms of respiratory status, overall performance status, and laboratory parameters of immune and inflammatory status. Adapt criteria with IRB approval as natural history and other treatments of COVID19 evolve. Carefully track number of eligible patients consented to the study receiving treatment Intent-to-treat analysis essential |
Stratification much like the PREVENT trial likely useful to match controls better. Examples are the Charlson Comorbidity Index (67) and the Wuhan/Guangdong Risk Score (68) Assess both rate of clinical decline in addition to net scores Randomize patients to LDRT or control arm a few hours prior to availability of linear accelerator to deliver radiation. This will reduce number of patients randomized to LDRT that deteriorate without receiving the experimental LDRT |
|
| Exclusion | Criteria should be clearly specified Some discussants felt that trials of LDRT are currently not indicated, due to lack of data on mechanism and shifting standard care (e.g., Dexamethasone use making value of immune markers potentially challenging) |
Ethical review- Institutional Review Board or another similar panel necessary | |
| Gender & minority inclusion | Inclusion particularly relevant with the epidemiology of infection. Data should be collected regarding social and economic status, living conditions, co-morbidities and patient geography (e.g., pollution and lung disease correlations, etc.) |
Underlying disparities in genetics or biology may impact outcome | |
| Trial type and projected number of patients, duration of study | |||
| Phase 1 | Generally, this is a specified Phase 1 trial or a run-in part of a Phase 2 | Intent-to-treat analysis is essential | |
| Phase 2, single arm or randomized | Run-in and randomization favored | Phase 2 run-in favored when there are Phase 1 safety data available | |
| Phase 3 | Felt to be insufficient evidence at present for a Phase 3 trial | ||
| Treatment | |||
| Radiation, target, dose, and schedule | Dose range of 0.35– 1.5 Gy (or up to 2 Gy); Single dose due to complex logistics Need to standardize definition of low, ultra-low, etc. |
Megavoltage recommended Needs better biological assessment of intention of chosen dose because different doses may affect different subsets of the COVID-19 immune response and may vary from person to person |
|
| Concomitant drugs required as part of treatment | Critical to include data on corticosteroids,; other antivirals, and other inflammatory agents Consider anticoagulants, oxygen use and status, cardiac inotropes, history of prior and current use of ACE inhibitors, diabetes Complex geographic and environmental interactions linked to lung disease in addition to smoking and mining(69) |
Describe/examine course of drugs in relation to LDRT because the relative timing of interventions could theoretically cause different therapeutic maneuvers to block each other. This may vary by economic situation (e.g., Low to Middle Income Country) |
|
| Remdesivir(70) and Dexamethasone(10) are baseline for all patients at present | |||
| Other drugs | Details of prior/ current medications | Timing of course in relation to LDRT | |
| Excluded | In this early phase it was felt that recruitment of pediatric/juvenile/middle-age and pregnant patients be avoided | No formal age cut-off was selected. Risk for secondary cancers made most feel age consideration to be reasonable |
|
| Drug - if randomized | Radiation was felt to be the primary agent to randomize (e.g., comparing different radiation doses or “standard of care” +/− radiation) Steroid dose could be added if necessary (or frequency of its use) |
Radiation (dose) +/− steroids vs. steroids without radiation More than one radiation dose could be tested vs. arm without radiation |
|
| Drug #1 | One participant mentioned concomitant use of antibodies to reduce selected cell populations and minimize cytokine storm as a possible treatment | No specific recommendation by workshop | |
| Response criteria | |||
| Circulating blood counts | Include granulocytes and lymphocytes, with a focus on subtyping via flow cytometry to evaluate subtypes (exhaustion, etc.) Mast cell populations are also of interest T cell lymphopenia and exhaustion, N:L ratio, HLA-DR + monocytes, CD14+:CD16+ monocytes, TF+ monocytes |
Neutrophil/lymphocyte ratio may be a helpful biomarker Dexamethasone may reset current trial designs in this context |
|
| Immunological- general | Pre- and post-treatment evaluation | Involvement with appropriate expertise. Dexamethasone may reset the current trial designs in this context | |
| Fibrosis biology felt to be important for both control and treatment patient cohorts | |||
|
Evidence of: Pro: antifibrotic effect |
Con - no anti- fibrotic effect | ||
| Normalization: IL-6, ratio of IL-10: TNF-α, CRP, IL-10/23, IL-1β, IFN-type I, IL-2, fibrinogen, TPA, PAI-1, ferritin, albumin, D-dimer, LDH, prothrombin, C3/C5 complement | Further rise in systemic proinflammatory markers, coagulopathy and immune paralysis | ||
| Immunological- SARS-CoV-2 related (See Table 2) | As above, other factors to be collected from the same samples as allowed (complement, IL-6, other IL factors, TNF-factors, etc.) | As above-appropriate expertise | |
|
Evidence of: Pro: improvement of viral disease |
Con: no improvement | ||
|
Normalization: IFN-α (or IP-10), Ab titers, S-protein tetramers |
Rising angiotensin II (viral load and lung injury) | ||
| Chemistry | AST, ALT and others per routine care | ||
| Imaging | |||
| Chest (CT, X-ray) | Chest X-ray, unilateral or bilateral changes or 3-D ultrasound if no X-ray available CT-scans likely difficult due to logistics involving movement of patients, possible contamination of equipment and staff exposures |
Lung infiltration may impact radiation lung dose | |
| Other organs | Liver, mediastinal content, scatter dose to the thyroid, stomach, colon, the great vessels, the bones of the chest including their marrow, and potentially other organs. | Not known if LDRT would be equally effective if blocking at the diaphragm is used rather than at the true inferior limit of the lungs | |
| Symptomatic response | |||
| System | Subjective and objective measures | Some felt symptomatic relief useful but not sufficient endpoint | |
| Patient outcomes | |||
| ICU-care required, or no longer required | Outcome measurements that are validated for the acute phase of therapy. | Hospital costs and outcomes related to ICU admission timing will vary situationally Acute medical management phase measurements like more rapid ICU discharge need to be used cautiously as it is unknown how these may correlate with severe late effects; i.e., early “benefits” that alter hospital management may not predict late functional outcome which may be more important |
|
| Duration in ICU (pre- and post LDRT) | Important parameter to consider as a primary trial efficacy endpoint | Changing acute management with LDRT might not correlate with ultimate pulmonary function | |
| Alive, duration | Long-term data should be sought | ||
| Death, when post LDRT | Possible co-primary endpoint with ICU duration | ||
| Long-term survivors | For survivors, evaluation of the long-term symptoms from viral infection, treatment, and the neutralizing capacity of antibodies | Can LDRT degrade development and maintenance of long-term immunity for survivors? Research subjects should be followed for the development of cancer and cardiovascular disease |
|
In considering the cytokine profile of COVID-19, the lack of type 1 interferon (IFN) production in response to SARS-CoV-2 infection is striking. Other coronaviruses are known to interfere with IFN synthesis (44), and SARS-CoV-2 may have similar attributes. Since Type 1 IFNs are important for antigen presentation, viral impairment of IFN production could have profound effects on development of adaptive anti-viral immunity and allow unrestricted viral replication. Recently, IFN-alpha has shown promise, either alone or with HIV-specific protease inhibitors, in treating coronavirus infections, including COVID-19 (45).
Myeloid cells appear to be dysregulated in COVID-19 and, although reports are inconsistent, increases in pro-inflammatory monocytes that produce high levels of IL-6 and other cytokines and have low MHC class-II expression may be related to dysfunctional innate and adaptive immunity and disease status (46). Dysfunction of alveolar macrophage by SARS-CoV-2 has also been proposed as a driver of the cytokine storm.(47) Other features of the disease that correlate with its severity are T and NK cell depletion and exhaustion (48). Along with increases in myeloid suppressor cells (49), these outcomes contribute to a high neutrophil to lymphocyte ratio (41).
SARS-CoV2 infection evolves rapidly, as will the microenvironment within the tissue to be irradiated with LDRT. Understanding the time-dose dependency of LDRT effects will be very challenging but crucial for dissecting LDRT’s therapeutic potential, if any. Many questions about the anti-inflammatory action of LDRT in infectious diseases, therefore, remain, and appropriate animal models of COVID-19 are urgently needed.
Risks
Previous studies with mouse models using combined radiation and influenza A exposure led to the hypothesis that prior lung irradiation might increase severity/susceptibility to SARS-CoV-2 and elevate the risk of pulmonary fibrosis (50). Prior SARS-CoV-2 exposure and recovery may also promote sensitivity and susceptibility to RT-mediated lung injury and fibrosis in cancer patients. Notably, increased morbidity and mortality from SARS-CoV-2 infection was reported among lung cancer or lung metastasis patients in a multicenter study while cancer patients without lung metastasis had no statistically significant differences compared to COVID-19 patients without cancer (51). COVID-19 patients with a history of lung RT had a poor prognosis and higher mortality risk with a mathematical survival model having a nearly linear relationship between mortality risk after COVID-19 diagnosis and mean lung RT dose (52).
LDRT includes exposure of whole lungs to radiation. Data from survivors of the atomic-bombings in Japan, who had average organ doses of about 0.2 Gy, indicate that the lifetime risk of lung cancer is higher in females than in males, the excess risk at 1 Sv for acute exposures being 2.6%–6.7% vs. 1.3%–2.0%, respectively (6). These ranges reflect differences in age at exposure for adults, statistical and dosimetry errors and the use of absolute vs relative risk projection/transfer models.
Other epidemiological studies provide similar estimates (6). The risk of fatal cancer and circulatory diseases after 1 Gy acute exposure is estimated by modeling at about 2 to 6% (3, 53, 54), with morbidity risk about 2-fold higher. Smoking has a major effect on risk, as does inflammation. Overall COVID-19 mortality is <5%, with a higher loss of life expectancy than death from radiation-induced cancer or circulatory disease. However, persons above age 70 y may have a much higher risk than 5%. Risk estimates for adverse events from LDRT should be weighed against other treatment approaches.
Overall perspectives
While there was a general consensus that LDRT for COVID-19 should be utilized only within a framework of rigorous and well-designed clinical studies, there was no agreement on whether safety data are sufficient and whether there is adequate rationale to proceed with clinical trials. Placing the role of LDRT in context of safe and effective vaccines as well as improved anti-viral and anti-inflammatory strategies is essential.
Although the workshop did not reach consensus on whether or not clinical trials of LDRT for COVID-19 are appropriate, based on biological knowledge and clinical experience to date with the pandemic and LDRT to treat COVID-19, one major goal of the workshop was to suggest guidelines to consider for a well-designed clinical study. Taking into consideration the pros and cons of many parameters, a possible framework for a protocol was developed (Table 3). However, this framework is not a blueprint; rather, it provides guidance for those who are committed to testing LDRT as a treatment option for the COVID-19 public health crisis.
Conclusions
It is clear that more preclinical data, ideally derived from a robust model reflective of SARS-CoV-2 pathophysiology, are needed, and that little data exist regarding the pathophysiological effects of treating a lung with LDRT in the midst of a viral vasculopathy and pneumonia. However, the efforts of radiation biologists to reveal the potential mechanistic interplay between radiobiologic response and virus-induced cytokine response in a target organ was recognized. Without supporting or refuting LDRT for COVID-19 as a treatment, this review intends to assist clinicians and researchers who plan to conduct preclinical and clinical studies, given the limited evidence on the safety and efficacy of LDRT for COVID-19. Recognizing that improved treatments for severely ill patients with COVID19 are necessary, any trial that is performed must be done cautiously, with full appropriate IRB oversight and must be cognizant of rapid changes in the biological understanding of the phases of the illness and the standard of care in this patient cohort. Also, the workshop participants recognize the potential of these novel trials to enhance understanding of the effects of radiation in modulating host immunity and anti-inflammatory response, which is why inclusion of biomarkers in all studies is crucial. Workshop participants spanned the entire arc of opinions from for to agnostic/neutral to against the clinical application of LDRT while generally agreeing that collaborative pre-clinical research and rapid reporting of results from any clinical trials are necessary.
Search strategy and Selection Criteria.
Two authors independently searched the clinicaltrials.gov for currently registered clinical trials using “Low Dose Radiation Therapy” and the PubMed using MeSH terms “Arthritis / radiotherapy; Inflammation / radiotherapy; Ionizing radiation / anti-inflammatory effects; Pneumonia / radiotherapy; Germany / radiotherapy; Radiotherapy dosage; Radiation injury / blood; Oxidative stress / radiotherapy; COVID-19 / SARS-CoV-2 / lung innate immunity; Coronavirus infection / blood; Betacoronavirus / blood coagulation; Coronavirus infection / pathology; Cytokine release syndrome; Encephalomyelitis, autoimmune, experimental / gamma rays; Alzheimer’s disease / radiation; Interleukin-6 / radiation; Cytokines / low-dose radiation; Nrf-2 / radiation; Myeloid cells / low-dose radiation” for published literature from 1999–2020 for this review, prepared as the Workshop Report on LDRT for COVID-19.
Acknowledgements
This work is supported by the National Council on Radiation Protection and Measurements (NCRP), Bethesda, MD, USA; the National Cancer Institute’s Radiation Research Program, and the National Institute of Allergy and Infectious Diseases’ Radiation and Nuclear Countermeasures Program, National Institutes of Health, Bethesda, MD, USA. NCRP acknowledges funding from the Centers for Disease Control and Prevention (CDC) through Grant No. 5NUE1EH001315. DGK is supported by R35CA197616 from the National Cancer Institute. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views and the opinions of the Institutes/organizations they represent.
Footnotes
Declaration of interests
The organizers of the workshop and lead authors of the manuscript, Drs. Held, Woloschak, Prasanna, Ahmed, Vikram, Coleman, and DiCarlo have nothing to disclose and performed this work in the interest of the general public. Drs. Buchsbaum, Cucinotta, Hu, Krishnan, Leitner, Marples, McBride, Rafii, Schaue, Sharon, and Sullivan have nothing to disclose. Dr. Chakravarti reports grants from Varian Medical Systems, from null, outside the submitted work. Dr. Formenti reports grants from Bristol Myers Squibb, Varian, Eli-Lilly, Janssen, Regeneron, Eisai, Merck, other from Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, Medlmmune, Merck US, EMD Serono/Merck, Accuray, other from Pfizer, outside the submitted work. Dr. Guha reports grants and personal fees from Johnson and Johnson, personal fees from FUSF, outside the submitted work. Dr. Khan has a patent Provisional pending. Dr. Kirsch reports other from Lumicell, other from Lumicell, other from Lumicell, other from Lumicell, other from Xrad Therapeutics, other from Xrad Therapeutics, other from Xrad Therapeutics, grants from Merck, grants from Eli Lilly, grants from Bristol-Myers Squibb, grants from Varian Medical Systems, outside the submitted work; In addition, Dr. Kirsch has a patent Radiosensitizers issued to XRad Therapuetics, a patent Imaging device licensed to Lumicell, and a patent Imaging agent with royalties paid to Lumicell. Dr. Mehta reports personal fees from Abbvie, personal fees from Celgene, personal fees from Blue Earth Diagnostics, personal fees from Astra Zeneca, personal fees from Tocagen, personal fees from Karyopharm, personal fees from Mevion, personal fees from Oncoceutics, outside the submitted work. Dr. Weichselbaum reports grants from Varian Medical Systems, grants from Regeneron, during the conduct of the study; other from Boost Therapeutics, other from Coordination Pharmaceutoicals Inc., other from ImmunoVir LLC, other from Magi Therapeutics, other from Oncosenescence, other from RefleXion Pharmaceuticals, other from AstraZeneca, other from Coordination Pharmaceuticals, other from Genus, other from Merck Serono S.A., other from Nano Proteagen, other from NK Max America Inc, personal fees from Boehringer Ingelheim, personal fees from Astrazeneca, personal fees from Merck Serono S.A., outside the submitted work; In addition, Dr. Weichselbaum has a patent Methods and Kits for Diagnosis and Triage of Patients with Colorectal Liver Metastases pending.
Contributor Information
Pataje G. Prasanna, National Cancer Institute, Bethesda, MD.
Gayle E. Woloschak, Northwestern University School of Medicine, Chicago, IL
Andrea L. DiCarlo, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Jeffrey C. Buchsbaum, National Cancer Institute, Bethesda, MD
Dörthe Schaue, University of California at Los Angeles, LA
Arnab Chakravarti, Ohio State University, James Comprehensive Cancer Center, Columbus, Ohio
Francis A. Cucinotta, University of Nevada Las Vegas, Las Vegas, NV, USA
Silvia C. Formenti, Weill Cornell Medicine, New York, NY
Chandan Guha, Albert Einstein College of Medicine, Bronx, NY
Dale J. Hu, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Mohammad K. Khan, Emory University School of Medicine, Winship Cancer Institute, Atlanta, GA
David G. Kirsch, Duke University School of Medicine, Durham, NC
Sunil Krishnan, Mayo Clinic, Jacksonville, FL
Wolfgang W. Leitner, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Brian Marples, University of Rochester Medical Center, Rochester, NY
William McBride, University of California at Los Angeles, LA
Minesh P. Mehta, Miami Cancer Institute, Miami, FL
Shahin Rafii, Weill Cornell Medicine, New York, NY
Elad Sharon, National Cancer Institute, Bethesda, MD
Julie M. Sullivan, U.S. Food and Drug Administration, Silver Spring, MD
Ralph R. Weichselbaum, University of Chicago Medicine and Ludwig Center for Metastasis Research, Chicago, IL
Mansoor M. Ahmed, National Cancer Institute, Bethesda, MD
Bhadrasain Vikram, National Cancer Institute, Bethesda, MD
C. Norman Coleman, National Cancer Institute, Bethesda, MD
Kathryn D. Held, National Council on Radiation Protection and Measurements, Bethesda, MD and Massachusetts General Hospital/Harvard Medical School, Boston, MA
References
- 1.Ameri A, Rahnama N, Bozorgmehr R, Mokhtari M, Farahbakhsh M, Nabavi M, et al. Low-dose whole-lung irradiation for COVID-19 pneumonia: Short course results. Int J Radiat Oncol Biol Phys. 2020; 10.1016/j.ijrobp.2020.07.026. [DOI] [PMC free article] [PubMed]
- 2.Hess CB, Buchwald ZS, Stokes W, Nasti TH, Switchenko JM, Weinberg BD, et al. Low-dose whole-lung radiation for COVID-19 pneumonia: planned day 7 interim analysis of a registered clinical trial. Cancer [Internet]. 2020; 10.1101/2020.06.03.20116988:[In press p.]. [DOI] [PMC free article] [PubMed]
- 3.Kirsch DG, Diehn M, Cucinotta FA, Weichselbaum R. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother Oncol. 2020; 147:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomaa S, Cardis E, Bouffler SD, Atkinson MJ, Hamada N. Low dose radiation therapy for COVID-19 pneumonia: is there any supportive evidence? Int J Radiat Biol. 2020:1–4. [DOI] [PubMed] [Google Scholar]
- 5.NCRP. Approaches for integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment. Bethesda, MD: National Council on Radiation Protection and Measurements; Report No. 186; 2020. [Google Scholar]
- 6.UNSCEAR. Effects of ionizing radiation. UNSCEAR 2006 report to the general assembly with scientific annexes. Volume I: report to the general assembly, scientific annexes A and B. New York, NY: United Nations Scientific Committee on the Effects of Atomic Radiation.; 2008. [Google Scholar]
- 7.Correll HL, Cowan II. Primary atypical pneumonia; analysis of therapeutic results in 155 cases. US Nav M Bull. 1943; 1943:980–7. [Google Scholar]
- 8.Oppenheimer A Roentgen therapy of “virus” pneumonia. American Journal of Roentgenology and Radiation Therapy. 1943; 1943:635–8. [Google Scholar]
- 9.Kirsch DG, Diehn M, Cucinotta FA, Weichselbaum R. Response letter: Radiation therapy for COVID-19 pneumopathy. Radiother Oncol. 2020; 149:238–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 11.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - Preliminary report. N Engl J Med. 2020. [DOI] [PubMed]
- 12.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselet B, Sonveaux P, Baatout S, Aerts A. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 2019; 76:699–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A. 1997; 94:6432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildebrandt G, Maggiorella L, Rodel F, Rodel V, Willis D, Trott KR. Mononuclear cell adhesion and cell adhesion molecule liberation after X-irradiation of activated endothelial cells in vitro. Int J Radiat Biol. 2002; 78:315–25. [DOI] [PubMed] [Google Scholar]
- 17.Hallahan DE, Virudachalam S, Kuchibhotla J. Nuclear factor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res. 1998; 58:5484–8. [PubMed] [Google Scholar]
- 18.Wilson DG, Mehta MP, Welsch JS, Chakravarti A, Rogers CL, Fontanesi J Investigating low-dose thoracic radiation as a treatment for COVID-19 patients to prevent respiratory failure. Radiat Res. 2020; 194:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch DG. Radiation therapy as a treatment for COVID-19? Int J Radiat Oncol Biol Phys. 2020. [DOI] [PMC free article] [PubMed]
- 20.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020; 20:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyamitra MM, DiCarlo AL, Taliaferro L. Understanding the pathophysiology and challenges of development of medical countermeasures for radiation-induced vascular/endothelial cell injuries: Report of a NIAID workshop, August 20, 2015. Radiat Res. 2016; 186:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesulu BP, Mahadevan LS, Aliru ML, Yang X, Bodd MH, Singh PK, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci. 2018; 3:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese EJ, Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013; 86:555–70. [PMC free article] [PubMed] [Google Scholar]
- 24.Seegenschmiedt MH, Micke O, Muecke R, German Cooperative Group on Radiotherapy for Non-malignant D. Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br J Radiol. 2015; 88:20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trott KR, Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother Oncol. 1999; 51:197–203. [DOI] [PubMed] [Google Scholar]
- 26.Sallenave JM, Guillot L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in Covid-19: Key therapeutic targets? Front Immunol. 2020; 11:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshino H, Chiba K, Saitoh T, Kashiwakura I. Ionizing radiation affects the expression of Toll-like receptors 2 and 4 in human monocytic cells through c-Jun N-terminal kinase activation. J Radiat Res. 2014; 55:876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchini M, Marano G, Cruciani M, Mengoli C, Pati I, Masiello F, et al. COVID-19-associated coagulopathy. Diagnosis (Berl). 2020. [DOI] [PubMed]
- 30.Kennedy AR, Maity A, Sanzari JK. A review of radiation-induced coagulopathy and new findings to support potential prevention strategies and treatments. Radiat Res. 2016; 186:121–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008; 49:381–9. [DOI] [PubMed] [Google Scholar]
- 32.Weng L, Williams RO, Vieira PL, Screaton G, Feldmann M, Dazzi F. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann Rheum Dis. 2010; 69:1519–26. [DOI] [PubMed] [Google Scholar]
- 33.Tsukimoto M, Nakatsukasa H, Sugawara K, Yamashita K, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat Res. 2008; 170:429–36. [DOI] [PubMed] [Google Scholar]
- 34.Frey B, Gaipl US, Sarter K, Zaiss MM, Stillkrieg W, Rodel F, et al. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 2009; 42:346–8. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Nam Y, Kim C, Lee H, Hong S, Kim HS, et al. Neuroprotective and Anti-Inflammatory Effects of Low-Moderate Dose Ionizing Radiation in Models of Alzheimer’s Disease. Int J Mol Sci. 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceyzeriat K, Tournier BB, Millet P, Frisoni GB, Garibotto V, Zilli T. Low-Dose Radiation Therapy: A New Treatment Strategy for Alzheimer’s Disease? J Alzheimers Dis. 2020; 74:411–9. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka Y, Nakayama H, Yoshida R, Hirosue A, Nagata M, Tanaka T, et al. IL-6 controls resistance to radiation by suppressing oxidative stress via the Nrf2-antioxidant pathway in oral squamous cell carcinoma. Br J Cancer. 2016; 115:1234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020; 20:389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 40.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020; 2:e428–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020; 27:992–1000 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020; 214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang IC, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011; 7:e1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020; 395:1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020; 20:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020; 57:102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Y, Wei X, Guan J, Qin S, Wang Z, Lu H, et al. COVID-19 pneumonia: CD8(+) T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020; 218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19). Cell Death Differ. 2020. [DOI] [PMC free article] [PubMed]
- 50.Manning CM, Johnston CJ, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. Lung irradiation increases mortality after influenza A virus challenge occurring late after exposure. Int J Radiat Oncol Biol Phys. 2013; 86:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020; 10:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabarriti R, Brodin NP, Maron MI, Tomé WA, Halmos B, Guha C, et al. Extent of prior lung irradiation and mortality in COVID-19 patients with a cancer history. Adv Radiat Oncol. 2020; 10.1016/j.adro.2020.04.028. [DOI] [PMC free article] [PubMed]
- 53.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012; 120:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pham TM, Sakata R, Grant EJ, Shimizu Y, Furukawa K, Takahashi I, et al. Radiation exposure and the risk of mortality from noncancer respiratory diseases in the life span study, 1950–2005. Radiat Res. 2013; 180:539–45. [DOI] [PubMed] [Google Scholar]
- 55.Bao C, Tao X, Cui W, Yi B, Pan T, Young KH, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020; 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goncalves CA, Sesterheim P. Serum amyloid A protein has been undervalued as a biomarker of COVID-19. Diabetes Metab Res Rev. 2020:e3376. [DOI] [PMC free article] [PubMed]
- 57.Fukada A, Kitagawa Y, Matsuoka M, Sakai J, Imai K, Tarumoto N, et al. Presepsin as a predictive biomarker of severity in COVID-19: A case series. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 58.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020; 58:1021–8. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Xiang X, Ren H, Xu L, Zhao L, Chen X, et al. Serum Amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J Infect. 2020; 80:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma A, Cheng J, Yang J, Dong M, Liao X, Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Crit Care. 2020; 24:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skarstein Kolberg E ACE2, COVID19 and serum ACE as a possible biomarker to predict severity of disease. J Clin Virol. 2020; 126:104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strope JD, Pharm DC, Figg WD. TMPRSS2: Potential biomarker for COVID-19 outcomes. J Clin Pharmacol. 2020; 60:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun JK, Zhang WH, Zou L, Liu Y, Li JJ, Kan XH, et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY). 2020; 12:11287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020; 50:382–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C, Fei D, Li X, Zhao M, Yu K. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020; 46:1475–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Zhang Y, Mo P, Liu J, Wang H, Wang F, et al. Neutrophil to CD4+ lymphocyte ratio as a potential biomarker in predicting virus negative conversion time in COVID-19. Int Immunopharmacol. 2020; 85:106683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christensen DM, Strange JE, Gislason G, Torp-Pedersen C, Gerds T, Fosbol E, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med. 2020. [DOI] [PMC free article] [PubMed]
- 68.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, et al. A tool for early prediction of Severe Coronavirus Disease 2019 (COVID-19): A multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020; 71:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trupin L, Balmes JR, Chen H, Eisner MD, Hammond SK, Katz PP, et al. An integrated model of environmental factors in adult asthma lung function and disease severity: a cross-sectional study. Environ Health. 2010; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olender SA, Perez KK, Go AS, Balani B, Price-Haywood EG, Shah NS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]


