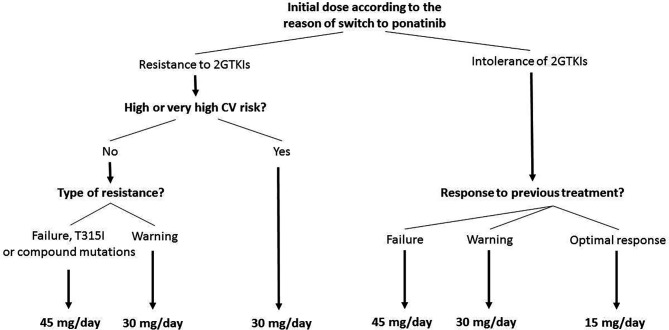
Figure 1.
Treatment algorithm for starting doses of ponatinib in eligible patients with chronic myeloid leukemia in chronic phase. In case of hematologic toxicity of 2nd generation tyrosine kinase inhibitors (2GTKIs), consider 15 mg/day as initial dose. Patients with a starting dose of 45 mg/day or 30 mg/day, should reduce to 15 mg/day upon achievement of a MR3 (MR2 in case of high or very high cardiovascular [CV] risk). Failure criteria defined according to the European LeukemiaNet (ELN) 2020 recommendations (BCR-ABL1 >10% within 1–3 months, >10% at 6 months, >1% at 12 months, and >1%, resistance mutations, high-risk additional chromosome abnormalities any time after 12 months) (7). Warning response defined according to the ELN 2020 recommendations (BCR-ABL1 >10% at 3 months, >1%–10% at 6 months, > 0.1%–1% at 12 months onwards) (7). Optimal response defined according to the ELN 2020 recommendations (BCR-ABL1 ≤10% at 3 months, ≤1% at 6 months, and ≤0.1% at 12 months onwards) (7). CV risk defined according to the 2016 European Guidelines on CV disease prevention (25).