Fig. 2.
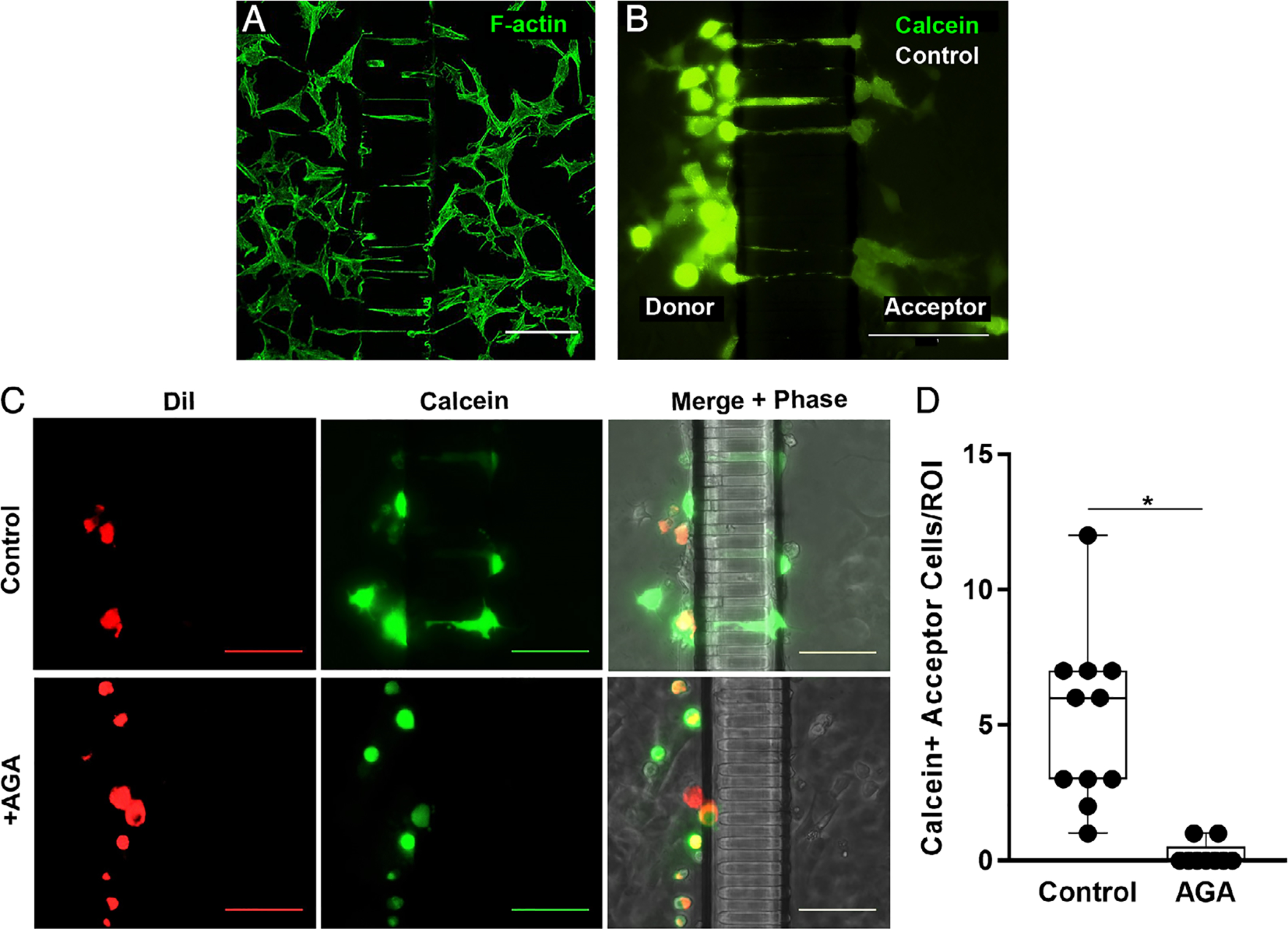
Osteocyte process ingrowth and gap junction functionality. (A) Confocal photomicrograph showing MLOY-4 osteocyte dendritic processes growing into the nanochannel array at 7 days culture in the Mμn device, visualized by AlexaFluor488-labeled phalloidin staining for F-Actin. (B) Parachute dye transfer assay image, showing Calcein-AM (green) transfer from donor compartment osteocytes to acceptor osteocytes in the “bystander” compartment of Mμn device. (C) Fluorescence and corresponding phase-contrast micrographs showing dye transfer across the channel array in osteocytes cultured under control conditions (top row), while no transfer to the acceptor side of the Mμn occurred when the connexin 43 blocker AGA was added, confirming gap junction communication between compartments. MLO-Y4 cells, labeled with gap junction permeable dye Calcein-AM (green) and gap junction impermeable membrane dye DiI (red), were parachuted onto one side of a previously MLO-Y4–seeded Mμn. Calcein-only labeled cells indicate gap junctional transfer from parachuted cells (donors) to cells on same side of the channel array, as well as across the channel array (acceptors) via first and second order network connections. (All scale bars =100 μm). (D) Number of Calcein-AM positive (Calcein+) cells on the acceptor side of the Mμn under control conditions and with AGA. (ROI = 100 μm on either side of channel array). n = 11 devices, *p = .0002, unpaired t test.
