Fig. 3.
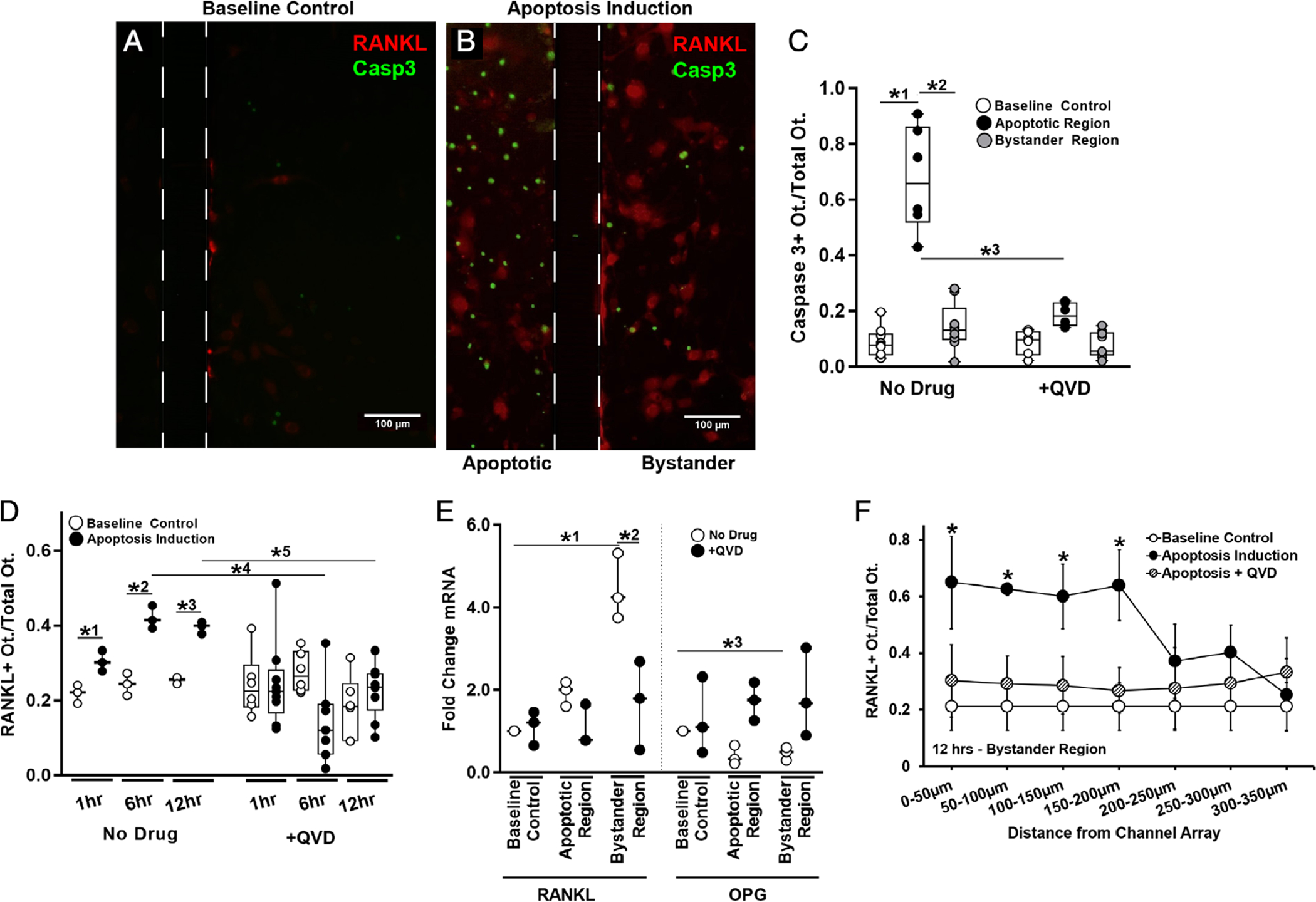
Osteocyte apoptosis and RANKL expression in the Mμn device. (A) Fluorescence photomicrograph of baseline control conditions for MLO-Y4 osteocytes cultured in the Mμn system, showing a few weakly fluorescent mCherry RANKL expressing osteocytes (red fluorescence). Boundaries of the nanochannel array outlined with a dotted line. Scale bar = 100 μm. (B) Fluorescence photomicrograph showing large numbers of apoptotic osteocytes (green fluorescence, shown using caspase 3 cleavage dye) at 12 hours after induction; apoptosis is effectively restricted to within the induction compartment of the Mμn device (on the left). The right side of the panel shows the opposite (non-stressed) compartment of the Mμn device, with large numbers of red fluorescing RANKL-expressing reporter osteocytes and a few apoptotic cells. (C) Quantification of the fraction of caspase 3–positive apoptotic cells before (baseline control) and in the apoptotic and bystander regions after apoptosis induction in the Mμn device. Data to the right are apoptosis data following addition of the apoptosis inhibitor QVD (10μM) 1 hour prior to apoptosis induction. Note that whereas almost 70% of osteocytes in the induction chamber undergo apoptosis (*1p = .0001 versus baseline control cells); there was no significant increase in osteocyte apoptosis in the bystander (non-stressed) compartment (*2p = .0001 versus apoptotic region and p = .32 versus baseline control), demonstrating that osteocyte apoptosis in the Mμn device is contained and separate. (D) Number of RANKL-expressing osteocytes in the bystander compartment was increased modestly at 1 hour (*1p = .01) and nearly twofold at 6 and 12 hours post-apoptosis induction (*2p = .0001 and *3p = .002, respectively). Inhibiting apoptosis with QVD prevented the increases in osteocyte RANKL expression in the bystander compartment at 6 and 12 hours (*4p = .001 and *5p = .003, respectively). n = 3 devices for control and n = 6 for QVD-treated groups. (E) With apoptosis induction, there was a more than fourfold increase in RANKL mRNA and a ~25% reduction in OPG mRNA in bystander osteocytes (*1p = .0001 for RANKL and *3p = .004 for OPG). Inhibiting apoptosis prevented the increase in RANKL gene expression (*2p < .001 versus apoptotic no drug) and the decrease in OPG mRNA level (p > .8 vs baseline for each condition), n = 3 devices per sample, tested in triplicate. (F) A high number of RANKL-expressing osteocytes extended ~200 μm from the nanochannel array/apoptotic signal source. Expression levels were low and homogeneous in the absence of apoptosis induced in the neighboring chamber and when treated with QVD. Comparison versus baseline control (*p = .043 at 0 to 50 μm, p = .0001 at 50 to 100 μm, and p = .02 at 100 to 150 μm and 150–200 μm), n = 9 devices. Data are shown as mean ± SD.
