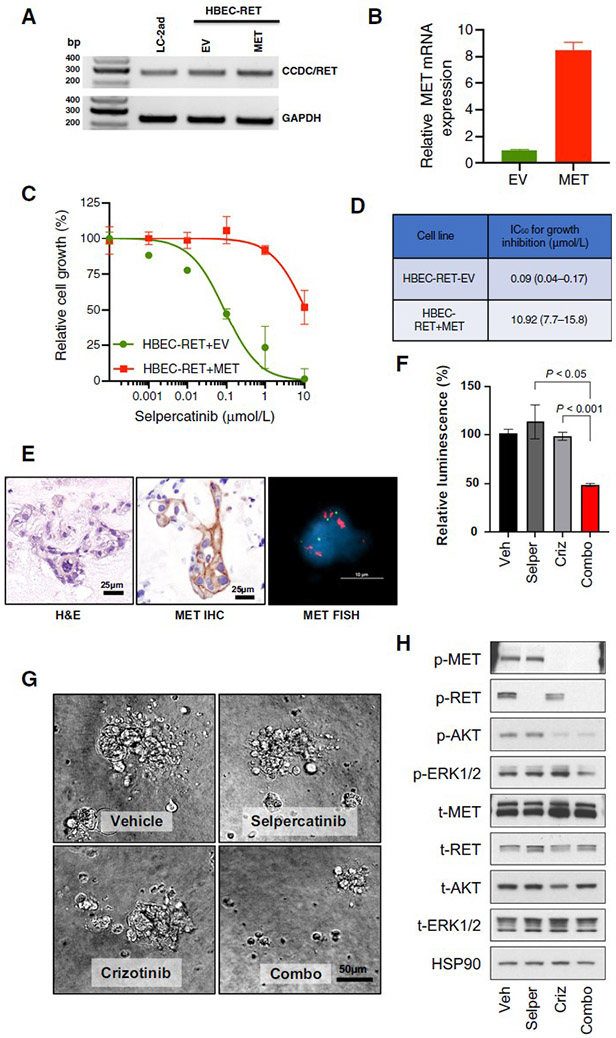
Figure 2. MET amplification drives resistance to selpercatinib and responds to MET inhibition in RET fusion-positive models.
A, B. RET fusion confirmed by RT-PCR using primers targeting CCDC6 (exon 1, forward) and RET (exon 12, reverse), and MET expression was confirmed by qPCR. C. Cells were treated with the indicated concentrations of selpercatinib for 96 hours and then the relative number of cells determined using proliferation dye. D. Viability data was analyzed and estimated IC50 values with the 95% confidence interval are shown. HBEC: bronchiolar epithelial cells. EV: empty vector. E. Patient-derived organoid from KIF5B-RET fusion-positive NSCLC (Case 2) shows MET gain by both IHC and FISH. F, G. Cell viability of dissociated cells from cultured organoids treated with either selpercatinib (0.3 μM) or crizotinib (1 μM) has little effect, but the combination is cytotoxic. H. Selpercatinib alone blocked RET activity whereas pAKT and pERK were retained, while combination treatment successfully led to inactivation of both AKT and ERK.