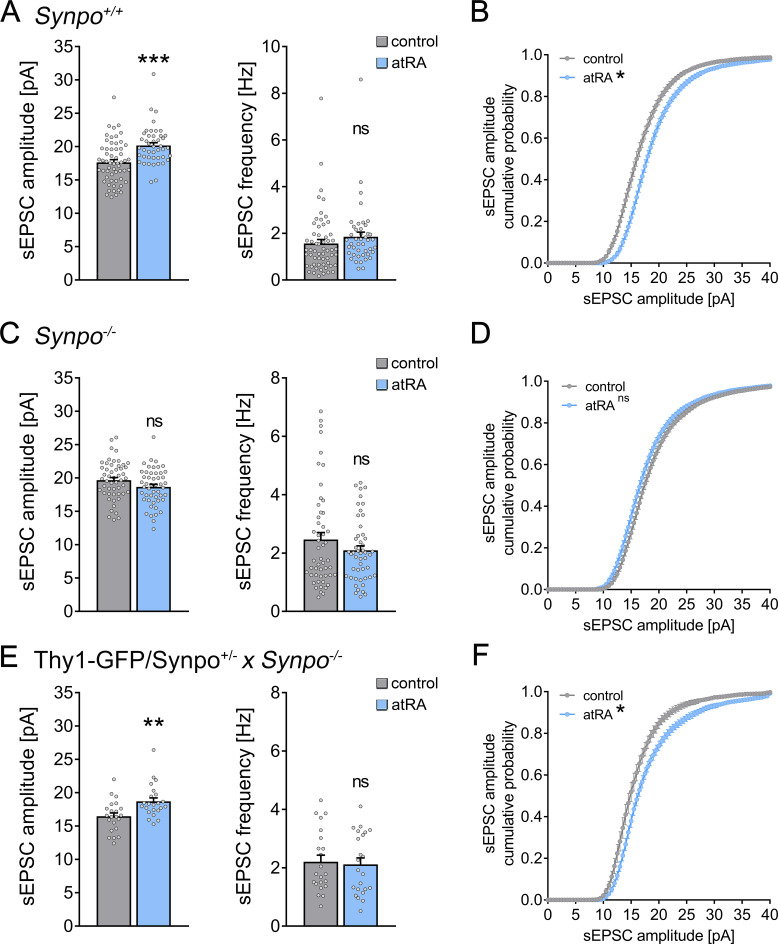
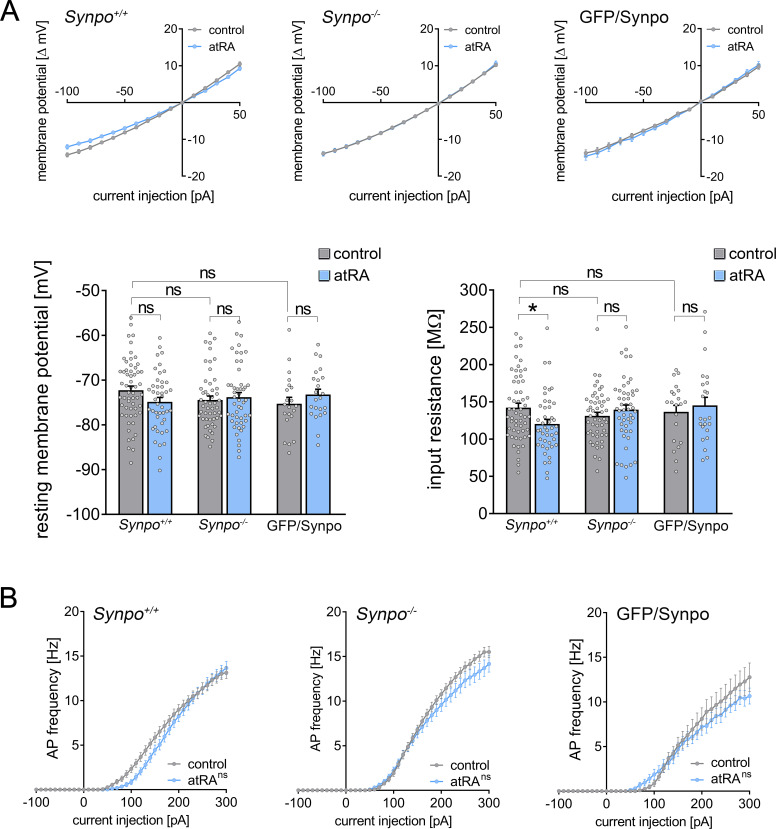
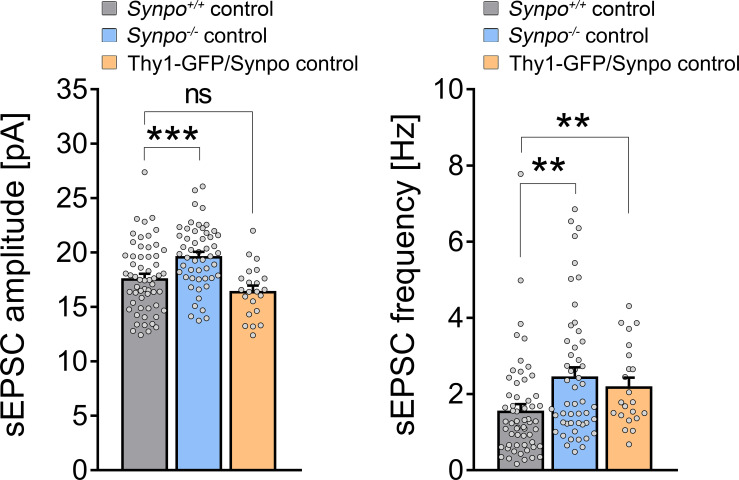
Figure 3. Effects of all-trans retinoic acid (atRA) in cortical slices prepared from synaptopodin-deficient mice.
(A, B) Group data (A) of AMPA receptor-mediated spontaneous excitatory postsynaptic currents (sEPSCs) recorded from superficial (layer 2/3) pyramidal neurons of the dorsomedial prefrontal cortex in slices prepared from wild-type animals (Synpo+/+) and cumulative distribution (B) of sEPSC amplitudes (ncontrol = 58 cells, natRA = 44 cells in seven independent experiments; Mann–Whitney test for column statistics, UsEPSC amplitude = 684; RM two-way ANOVA followed by Sidak’s multiple comparisons for statistical evaluation of cumulative sEPSC amplitude distributions). (C, D) Group data (C) of AMPA receptor-mediated sEPSCs recorded from superficial (layer 2/3) pyramidal neurons of the dorsomedial prefrontal cortex in slices prepared from synaptopodin-deficient mice (Synpo−/−) and cumulative distribution (D) of sEPSC amplitudes (ncontrol = 51 cells, natRA = 49 cells in seven independent experiments; Mann–Whitney test for column statistics and RM two-way ANOVA followed by Sidak’s multiple comparisons for statistical evaluation of cumulative sEPSC amplitude distributions). (E, F) Group data (E) of sEPSC recordings and cumulative distribution (F) of sEPSC amplitudes in cortical slices prepared from transgenic mice expressing GFP-tagged synaptopodin under the control of the Thy1.2 promotor on synaptopodin-deficient genetic background (Thy1-GFP/Synpo+/−x Synpo–/–; ncontrol = 22 cells, natRA = 23 cells in three independent experiments; Mann–Whitney test for column statistics, UsEPSC amplitude = 125; RM two-way ANOVA followed by Sidak’s multiple comparisons for statistical evaluation of cumulative sEPSC amplitude distributions). Individual data points are indicated by gray dots. Values represent mean ± s.e.m. (ns, non-significant difference, ***p<0.001, **p<0.01).