Figure 5 – Substrate peptide-kinase interactions and their allosteric modulation by small molecules probed using ER/K linkers.
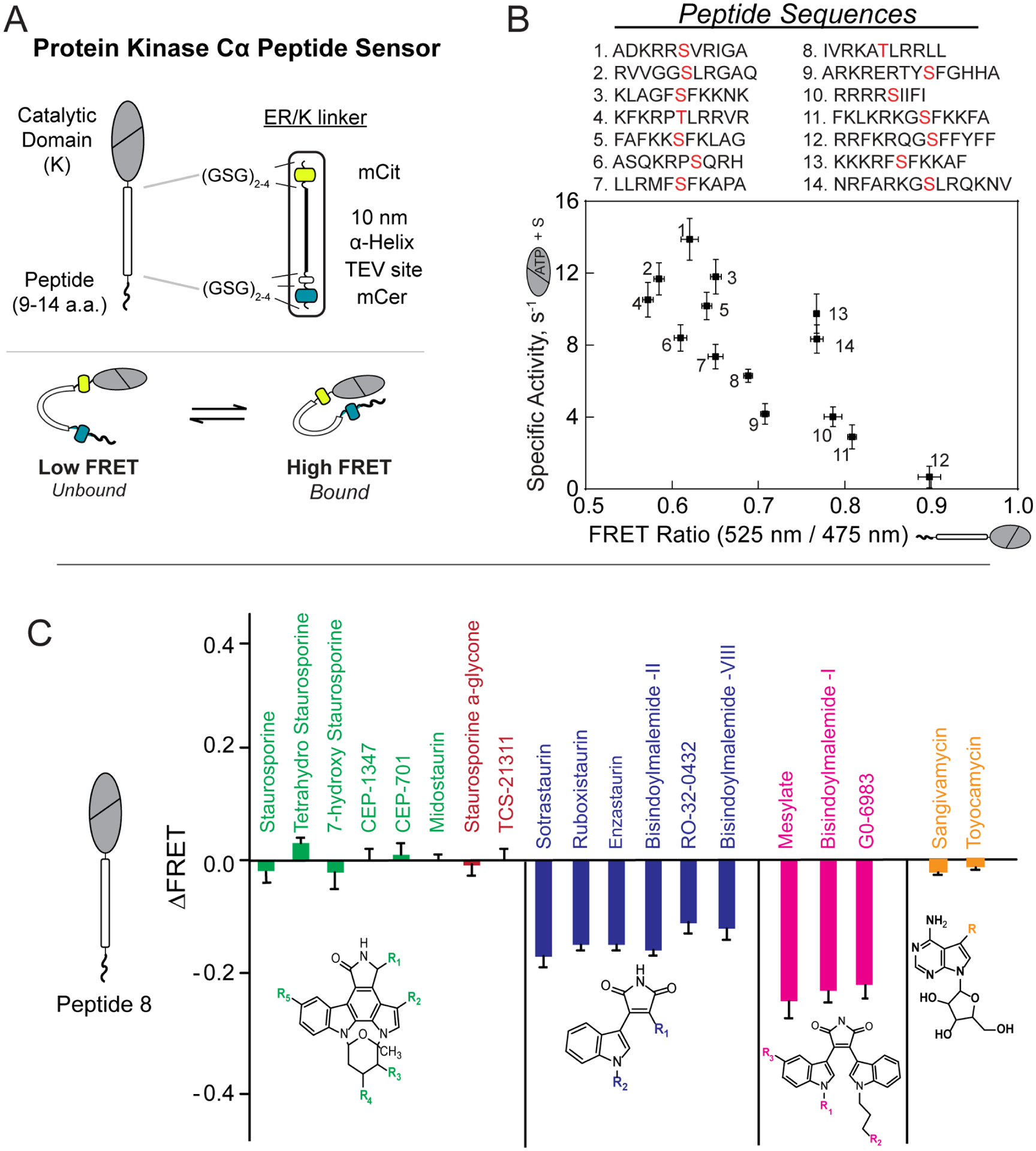
A) Cartoon schematic of PKC substrate peptide biosensor (top) and mechanism of action (bottom)
B) Correlation between kinase activity and FRET ratio observed for 14 different peptides derived from phosphorylated substrates of PKC (phosphorylated Ser/Thr residue is highlighted in red). Lower FRET ratios (affinities) for peptides were observed to correlate with higher activity.
C) Families of structurally similar small molecule kinase inhibitors led to similar observed changes in FRET ratio in PKC biosensors. Small molecules with a purported bitopic mode of binding (navy, magenta) were more effective in disrupting the kinase/substrate interaction.
For all experiments: N ≥ 3, and data are shown as mean ± SE.
Panels A and B adapted from Sommese et al., JBC 2016 This research was originally published in the Journal of Biological Chemistry. Substrate Affinity Differentially Influences Protein Kinase C Regulation and Inhibitor Potency. J Biol Chem. 2016; 291(42):21963–21970. © the American Society for Biochemistry and Molecular Biology
Panel C adapted with permission from (Ma et al., Biochemistry 2018). Copyright (2018) American Chemical Society
