Figure 2: Antiretroviral combination therapy decreases HML-2 levels, which correlates with a deceleration in clinical progression.
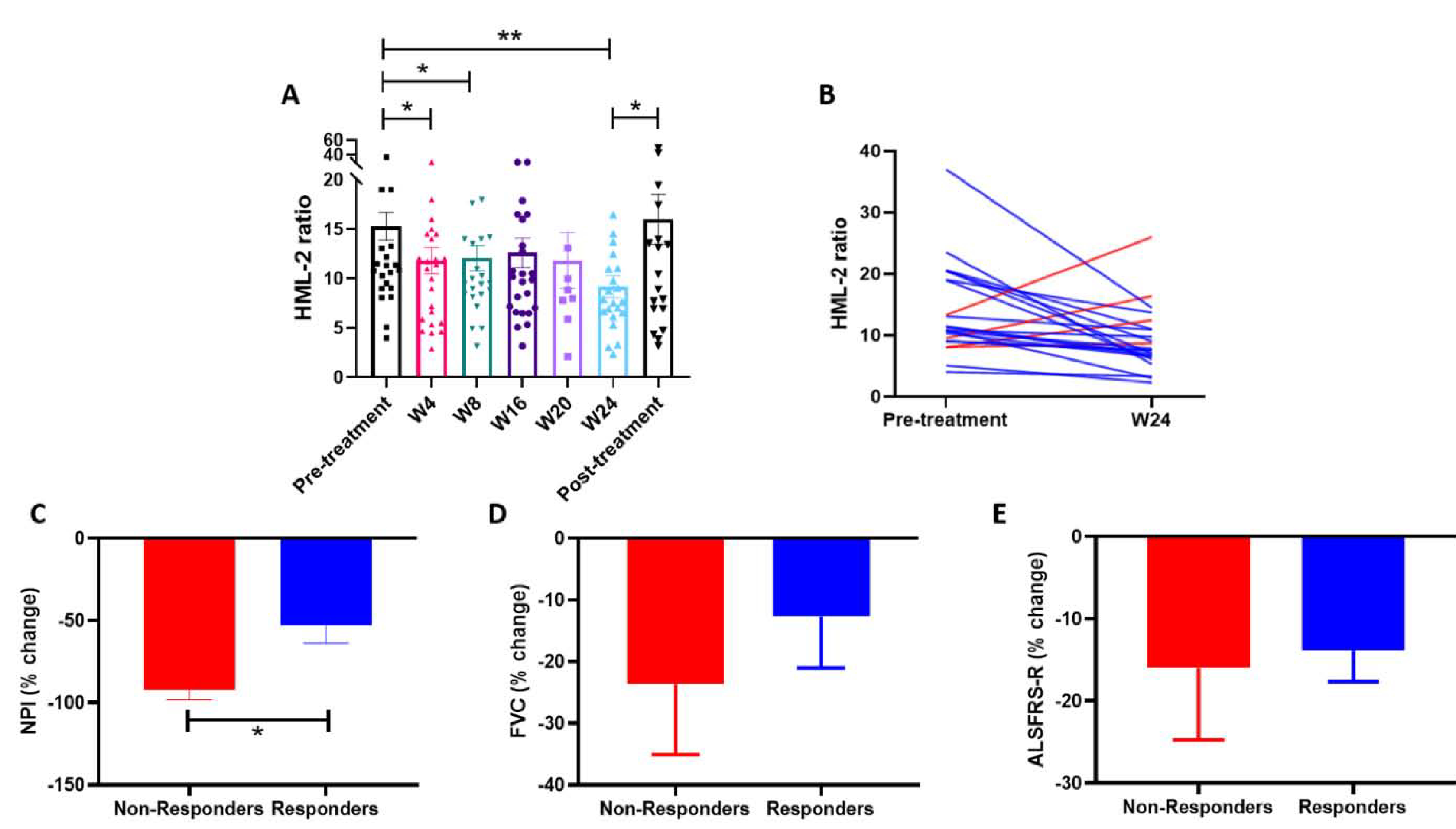
A) Mean HML-2 ratio as measured by ddPCR at baseline/screening (Pre-treatment) (n=29), at week 4 (n=26), week 8 (n=24), week 16 (n=26), week 20 (n=9) and week 24 of treatment (n=22), and one week after stopping treatment (Post-treatment) (n=23). B) HML-2 ratio at baseline/screening and at week 24. Blue lines represent responders and red lines represent non-responders. C, D and E) Comparison of the percent change in clinical scores from pre-treatment (mean baseline/screening) to week 24 between responders and non-responders. C) Percent change in NPI in responders and non-responders (*P=0.01). D) Percent change in FVC (% predicted) in responders and non-responders. E) Percent change in ALSFRS-R in responders and non-responders.
