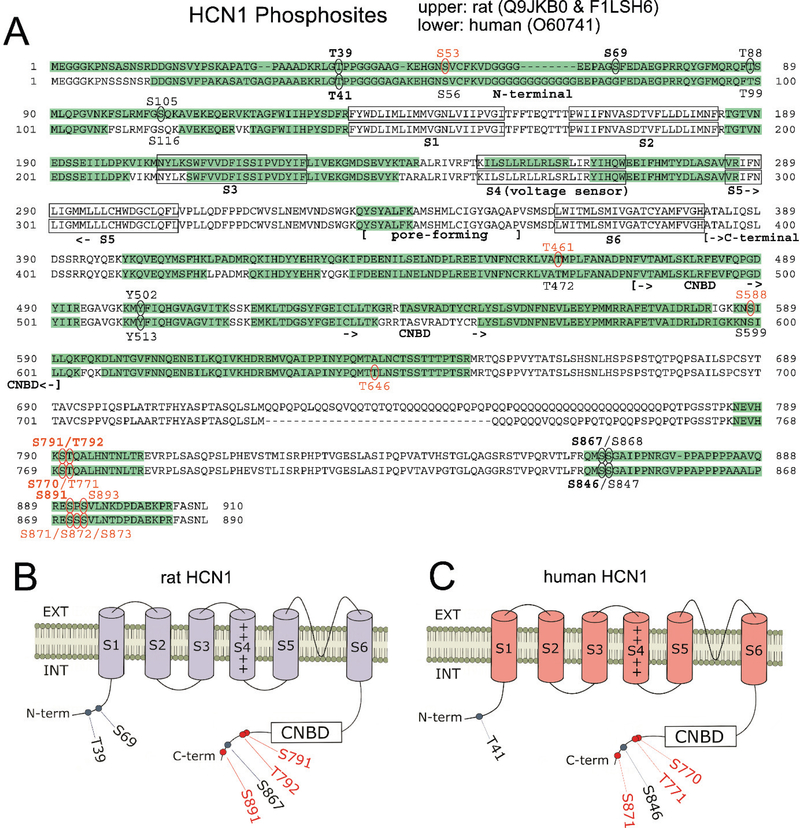
Figure 2.
Comparison of rat and human HCN1 phosphosites. (A) Amino acid sequences of rat and human HCN1 are juxtaposed. Green highlights show regions of each HCN sequence that were observed by mass spectrometry. Ovals indicate detected phosphosites; red labels indicate novel phosphosites; bold labels indicate phosphosites observed in ≥ 50% of either species’ samples. Structural features of HCN channels are denoted: the intracellular N-terminal region, the six transmembrane domains (S1-S6), the pore-forming region, and an extensive intracellular C-terminal region incorporating the cyclic nucleotide binding domain (CNBD). (B) Representation of the rat HCN1 channel showing phosphosites that were phosphorylated in at least 50% of rat samples (with red labeling denoting novel phosphosites). (C) Representation of the human HCN1 channel showing phosphosites that are homologous to the prevalent rat phosphosites shown in (B). Novel phosphosites are labeled in red.