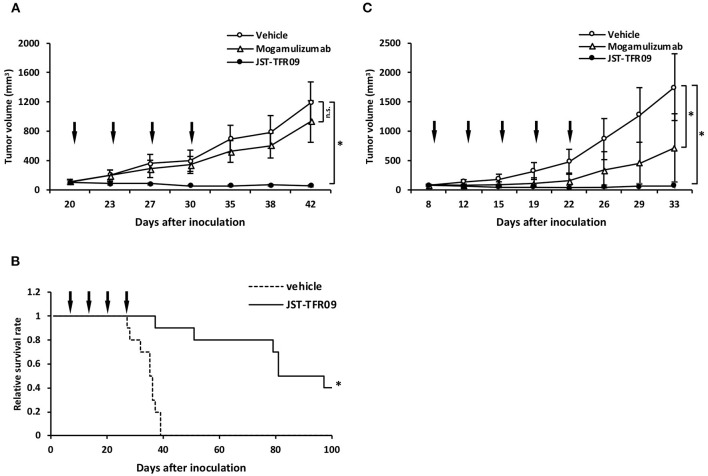
Figure 8.
Anti-tumor activity of the fully human JST-TFR09 antibody in xenograft mouse models. (A) ATLL HTLV-1-infected MT-2 s.c. tumor volumes in NOG mice treated with 10 mg/kg JST-TFR09, mogamulizumab, or vehicle control (i.v.). Arrows indicate the day of injection. *p < 0.05 compared to the vehicle control group. (B) Kaplan–Meier survival curves of NOG mice bearing MT-2 cells, treated with JST-TFR09 or vehicle control. *p < 0.05 (log-rank test) compared to the vehicle control. (C) Cutaneous T-cell lymphoma HH s.c. tumor volume in SCID mice treated with 20 mg/kg of JST-TFR09, mogamulizumab, or vehicle. The arrow indicates the time of antibody injection. *p < 0.05 compared to the vehicle control. Mogamulizumab was added as an antibody with known ADCC activity. This figure was reprinted from Shimosaki et al. (173) with permission from Elsevier.