Abstract
CD19-targeted chimeric antigen receptor T cells (CAR T cells) have shown excellent activity against relapsed and refractory (R/R) diffuse large B-cell lymphoma (DLBCL). CAR T cell therapy is associated with early toxicities, including cytokine release syndrome and neurotoxicity. The incidence and severity of these toxicities has in part been associated with baseline disease and patient characteristics, which may also impact overall survival (OS) and progression-free survival (PFS). However, there is limited data on patient selection and how to better predict toxicities or outcomes. Indexes used in DLBCL like the international prognostic index (IPI and age-adjusted IPI, aaIPI) and, in transplant, like the hematopoietic cell transplantation comorbidity index (HCT-CI) have not been evaluated in this setting. We evaluated 4 indexes (IPI, aaIPI, HCT-CI and the Charlson Comorbidity Index or CCI) and their associations with early CAR T cell related-toxicities and outcomes. We demonstrated an association between high-risk IPI or aaIPI and inferior PFS in patients with R/R DLBCL treated with CAR T cell therapies. We also found an association between aaIPI and IPI with OS and neurotoxicity, respectively. CCI was not associated with toxicities or outcomes and due to sample size, we could not conclude on associations with the HCT-CI. Both the IPI and aaIPI are widely used tools that can now provide better information to guide selection of patients who would best benefit from CD19 CAR T cell therapy.
Keywords: Chimeric antigen receptor T cells, cellular therapy, international prognostic index, non-Hodgkin lymphoma
INTRODUCTION:
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma (NHL) and represents up to 35% of adult NHL (1,2). While 10-year disease-free survival has been reported at 64%, outcomes after disease progression are poor, with 70% of deaths secondary to lymphoma within the first 2 years after disease progression (3,4). CD19-targeted chimeric antigen receptor T cells (CAR T cells) have shown excellent activity against B lymphoid malignancies, including relapsed/refractory (R/R) DLBCL (5–8).
The two most frequent early toxicities after the CAR T cell infusion are cytokine release syndrome (CRS) and neurotoxicity (9,10). CRS is a systemic inflammatory response triggered by in vivo CAR T cell expansion that can lead to fever, vascular instability, macrophage activation syndrome and multi-organ system toxicity (11). The severity of CRS is strongly associated with the severity of neurotoxicity. Neurological events may include headache, aphasia, tremor, encephalopathy, seizures or diffuse cerebral edema, among others. Severe CRS and neurotoxicity have been described with an incidence of 12–23% and 12–31%, respectively (12,13). There is currently limited published data that can guide patient selection for this treatment regarding both toxicities and outcomes. The international prognostic index (IPI) and the age-adjusted IPI (aaIPI) are two indexes used to identify DLCBL patients with a higher probability of survival (14). IPI has been validated for patients with DLBCL both in the frontline and salvage setting but not before CAR T cell therapy (15–17).
Salvage regimens for R/R DLBCL usually include consolidation with autologous stem cell transplantation (SCT)(18). In that context, the Charlson Comorbidity Index (CCI) (19) was adapted and modified for patients undergoing hematopoietic cell transplantation (HCT) and renamed the HCT comorbidity index (HCT-CI), which has demonstrated an association between prior organ dysfunctions and non-relapse mortality (NRM) or overall survival (OS) after HCT (20). Neither the HCT-CI nor CCI have been evaluated in patients receiving CAR T cell therapy. This study aims to evaluate a possible association between indexes routinely used in the care of patients with R/R DLBCL (IPI, aaIPI, CCI and HCT-CI) and early toxicities and overall outcomes in DLBCL patients treated with FDA-approved CD-19 directed CAR-T cell therapy.
METHODS:
Study population
Between February 2018 and June 2019, all consecutive adult patients (age ≥ 18 years old) diagnosed with R/R DLBCL who received FDA-approved CD19 CAR T cell products, axicebtagene ciloleucel (axi-cel, Yescarta, Kite/Gilead) or tisagenlecleucel (tisa-cel, Kymriah©, Novartis), at our center were included in this study. Written informed consent was obtained from all patients before CAR T cell infusions. Approval for this retrospective study was obtained from our Institutional Review and Privacy Board.
CAR T cell infusion and supportive care
According to the product label, patients treated with tisa-cel received a lymphodepleting regimen consisting of fludarabine 25 mg/m2 iv daily and cyclophosphamide 250 mg/m2 iv daily for 3 days; or bendamustine 90 mg/m2 iv for 2 days. Patients treated with axi-cel received fludarabine 30 mg/m2 iv and cyclophosphamide 500 mg/m2 iv for 3 days. All patients received supportive care and prophylaxis against opportunistic infections according to institutional guidelines.
Data collection and scoring
Data were collected from the institutional database and additional review of the electronic medical records. The IPI, aaIPI, HCT-CI and CCI were calculated as previously described (15,19,20), with the following modifications. The IPI and aaIPI were assessed based on serum lactate dehydrogenase (LDH), performance status and positron emission tomography (PET) imaging at the time of lymphodepletion, in order to have a homogeneous assessment of disease status given the wide spectrum of bridging therapies. Comorbidities were evaluated at the time of leukapheresis to calculate HCT-CI and CCI. Risk groups were stratified as in the original publications. Briefly, for the IPI, patients were classified into low-risk (IPI 0–1), low intermediate-risk (IPI 2), high intermediate-risk (IPI 3) and high-risk groups (IPI 4–5). For the aaIPI, patients were classified into low-risk (aaIPI 0), low intermediate-risk (aaIPI 1), high intermediate-risk (aaIPI 2) and high-risk groups (aaIPI 3). For the HCT-CI, patients were classified into low-risk (HCT-CI 0), intermediate-risk (HCT-CI 1–2) or high-risk groups (HCT-CI ≥ 3). For the CCI, patients were classified into low-risk (CCI 0), intermediate-risk (CCI 1) and high-risk groups (CCI ≥2). Patients without evaluable baseline disease status or pulmonary function tests (PFT) before cell infusion were excluded from the calculation of IPI and aaIPI or HCT-CI, respectively. Due to sample size, patients were clustered in two categories for each index except for the CCI: a low-risk category (including low and low intermediate-risk groups for the IPI and aaIPI (21); and low and intermediate-risk groups for the HCT-CI), and a high-risk category (including high intermediate and high-risk groups for the IPI and aaIPI; and high-risk group for the HCT-CI). PET imaging was performed after completing bridging therapy, at day 30 and at day 90 after treatment. Outcome data was assessed per last known follow-up.
Definitions and endpoints
The primary endpoint of this study was to determine the association of aaIPI, IPI, CCI and HCT-CI with progression-free survival (PFS) after the CAR T cell infusion. Secondary endpoints were OS and the ability of the various indexes to detect early CAR T cell-related toxicities. PFS was defined as time until progression, death or last follow-up and OS was defined as time until death or last follow-up. Early CAR T cell-related toxicity was defined as the incidence of CRS or neurotoxicity (according to the American Society of Transplant and Cellular Therapy grading system) (22) within the first 30 days after the CAR T cell infusion.
Statistical analysis
A descriptive statistical analysis was used to summarize patient and disease characteristics. The Kaplan-Meier method was used to calculate OS and PFS. Univariate Cox proportional hazard regression model was used to investigate the association between the prognostic indexes evaluated (IPI, aaIPI, HCT-CI and CCI), each individual variable that compose them and outcomes (PFS and OS). Competing risks regression was used to evaluate the prognostic indexes and their association with early CAR T cell-related toxicities (neurotoxicity and/or CRS), where death was used as a competing event. Multivariate analysis was performed with the index with the highest significance in the univariate analysis (aaIPI) for PFS and OS. This analysis was adjusted for sex and age as covariates. All statistical analyses were performed using R, version 3.6.0.
RESULTS:
Patient characteristics
Between February 2018 and June 2019, 60 consecutive adult patients with R/R DLBCL who received FDA-approved CD19 CAR T cell products at our center were included (Table 1). Median age at the time of CAR T cell infusion was 63 (19–86). The majority of the patients were male (70%), with good performance status (PS ≤1 86.7%) and advanced disease stage (stage III or IV 85.0%). The underlying disease was DLBCL for all patients (41.7% transformed DLBCL and 58.3% de novo DLBCL). Histological subtypes and distribution of comorbidities are shown in table 1.
Table 1.
Patient characteristics
| Characteristics | Axi-cel | Tisa-cel | All | |
|---|---|---|---|---|
| Number of patients | 43 (71.7%) | 17 (28.3%) | 60 (100%) | |
| Median age, years old (range) - Nº of patients ≤ 60 years old - Nº of patients > 60 years old |
61 (19–78) 20 (33.3%) 23 (53.4%) |
71 (45–85) 4 (23.5%) 13 (76.4%) |
63 (19–85) 24 (40%) 36 (60%) |
|
| Gender - Male - Female |
35 (81.4%) 8 (18.6%) |
7 (41.2%) 10 (58.8%) |
42 (70%) 18 (30%) |
|
| Performance status <1 | 36 (83.7%) | 16 (94.1%) | 52 (86.7%) | |
| Underlying disease - De novo DLBCL - Transformed DLBCL Histological subtypes - DLBCL, subtype Germinal Center B-cell - DLBCL, subtype Non-Germinal Center B-cell - High grade lymphoma B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements - Other subtypes |
24 (55.8%) 19 (44.2%) 19 (44.2%) 14 (32.5%) 5 (11.6%) 5 (11.6%) |
11 (64.7%) 6 (35.3%) 6 (35.3%) 10 (58.8%) 1 (5.9%) 0 (0%) |
35 (58.3%) 25 (41.7%) 25 (41.7%) 24 (40%) 6 (10%) 5 (8.3%) |
|
| Nº patients with calculable stage* - Stage III or IV |
38 35 (92.1%) |
14 10 (71.4%) |
52 45 (86.5%) |
|
| Elevated LDH | 24 (63.1%) | 6 (35.3%) | 30 (50%) | |
| Prior autologous transplant | 9 (20.9%) | 3 (17.6%) | 12 (20%) | |
| Prior allogeneic transplant | 5 (11.6%) | 0 (0%) | 5 (8.3%) | |
| Lymphodepleting regimen - FluCy - Bendamustine |
43 (100%) - |
14 (82.3%) 3 (17.6%) |
- |
|
| Comorbidities | Axi-cel (n=43) |
Tisa-cel (n=17) |
All patients (n=60) |
Calculable HCT-CI (n=15) |
| - Arrhythmia - Cardiac - Inflammatory bowel disease - Diabetes Mellitus ◦ Diabetes Mellitus with end organ damage - Cerebrovascular disease - Psychiatric disturbance - Hepatic impairment, mild - Obesity - Infection - Rheumatologic disorders - Peptic ulcer - Moderate/severe renal abnormalities - Chronic pulmonary disease1 - Moderate pulmonary1 - Prior solid tumor - Any solid tumor during the last 5 years - Heart valve disease - Severe pulmonary1 - Moderate/severe hepatic impairment |
5 (11.6%) 5 (11.6%) 0 (0%) 5 (11.6%) 2 (4.6%) 1 (2.3%) 6 (13.9%) 8 (18.6%) 7 (16.3%) 7 (16.3%) 4 (9.3%) 3 (7%) 0 (0%) 4 (9.3%) 2 (4.6%) 3 (7%) 2 (4.6%) 6 (13.9%) 4 (9.3%) 1 (2.3%) |
4 (23.5%) 3 (17.6%) 1 (5.9%) 1 (5.9%) 0 (0%) 1 (5.9%) 2 (11.8%) 2 (11.8%) 1 (5.9%) 2 (11.8%) 2 (11.8%) 1 (5.9%) 0 (0%) 0 (0%) 2 (11.8%) 3 (17.6%) 1 (5.9%) 3 (17.6%) 0 (0%) 0 (0%) |
9 (15%) 8 (13.3%) 1 (1.6%) 6 (10%) 2 (3.3%) 2 (3.3%) 8 (13.3%) 10 (16.6%) 8 (13.3%) 9 (15%) 6 (10%) 4 (6.6%) 0 (0%) 4 (6.6%) 4 (6.6%) 6 (10%) 3 (5%) 9 (15%) 4 (6.6%) 1 (1.6%) |
3 (20%) 2 (13.3%) 0 (0%) 3 (20%) 1 (6.6%) 1 (6.6%) 0 (0%) 3 (20%) 3 (20%) 1 (6.6%) 2 (13.3%) 1 (6.6%) 0 (0%) 2 (13.3%) 4 (26.6%) 0 (0%) 0 (0%) 2 (13.3%) 4 (26.6%) 0 (0%) |
Diagnosis based on clinical symptoms. DLBCL: Diffuse large B-cell lymphoma, LDH: Lactate dehydrogenase.
Seventeen patients had received a prior SCT (12 patients received a prior autologous SCT and 5 patients an allogeneic SCT). Axi-cel was the most commonly used CAR T cell product (71.6%) and the combination of fludarabine and cyclophosphamide (FluCy) was the most common lymphodepleting chemotherapy for both products. Baseline characteristics of patients receiving axi-cel and tisa-cel are described in table 1. The median follow-up was 206 days (24–562).
Prognostic indexes and risk stratification
We examined the associations of 4 indexes (IPI, aaIPI, HCT-CI and CCI) in patients receiving FDA-approved CD19 CAR T cell products with early CAR T cell related-toxicities and outcomes. Risk stratification for each index is summarized in table 2.
Table 2.
Prognostic scores
| N (%) | |
|---|---|
| IPI (n=52) - low-risk group - low intermediate-risk group - high intermediate-risk group - high-risk group Adapted categories - low-risk category - high-risk category |
8 (15.4%) 17 (32.7%) 20 (38.5%) 7 (13.5%) 25 (48.1%) 27 (51.9%) |
| aaIPI (n=52) - low-risk group - low intermediate-risk group - high intermediate-risk group - high-risk group Adapted categories - low-risk category - high-risk category |
4 (7.7%) 22 (42.3%) 22 (42.3%) 4 (7.7%) 26 (50%) 26 (50%) |
| HCT-CI (n=15) - low-risk group - intermediate-risk group - high-risk group Adapted categories - low-risk category - high-risk category |
3 (20%) 5 (33.3%) 7 (46.7%) 8 (53.3%) 7 (46.7%) |
| CCI (n=60) - low-risk group - intermediate-risk group - high-risk group |
34 (56.7%) 14 (23.3%) 12 (20%) |
IPI: International Prognostic Index, aaIPI: Age adjusted Intenational Prognostic Index, HCT-CI: Hematopoietic Cell Transplant Comorbidity Index, CCI: Charlson Comorbidity Index
PET imaging was performed after completing bridging therapy in all patients except 8. The remaining 52 patients had all risk factors included in the IPI and aaIPI available, and were included in the analysis. The median IPI and aaIPI score were 3 (0–5) and 2 (0–3), respectively.
PFTs were not routinely required prior to a CAR T cell treatment, so pulmonary impairment could not be formally evaluated in most patients. Consequently, only 15 patients were evaluable for all 17 comorbidities included in the HCT-CI. The prevalence of comorbidities is shown in table 1. Mild hepatic alterations were the most frequent comorbidity (10/15, 66.6%) followed by arrhythmia (9/15, 60%), heart valve disease (9/15, 60%) and infections (9/15, 60%). Other cardiac events, psychiatric disturbances and obesity were present in more than half of the evaluable population. Median HCT-CI score was 1 (0–9). Risk stratification at the time of leukapheresis was as follows, 3 (20%) were low risk, 5 (33.3%) were intermediate risk and 7 (46.7%) were high risk HCT-CI. Due to sample size, cases were grouped into two categories: a low-risk category (HCT-CI <3, n=8, 53.3%) and a high-risk category (HCT-CI ≥ 3, n=7, 46.7%). Since the HCT-CI could only be assessed in a minority of patients, we also evaluated the CCI. Each of the 19 comorbidities included in the CCI could be assessed in all 60 patients (table 1). Similar to the HCT-CI, mild liver disease was the most prevalent comorbidity (16.7%). Myocardial infarct, connective tissue disease and diabetes were present in 10%. Median CCI score was 0 (0–7). Thirty-five patients (58.3%) were low risk, 12 (20%) were intermediate risk and 13 (21.7%) were high risk.
Progression free and overall survival
At last follow-up, 33 patients were alive with no evidence of disease, and 41 patients were alive. The probability of PFS at 3 and 6 months was 68.3% (95% CI 57.5%−81.2%) and 59.5% (95% CI 48.2%−73.5%), respectively. The probability of OS at 3 months and 6 months for our series was 88.3% (95% confidence interval (CI), 80.6–96.8) and 76% (95% CI, 65.7–87.8), respectively.
In the univariate analysis, both IPI ≥ 3 and aaIPI ≥ 2 were associated with inferior PFS (HR 2.86 [95% CI 1.09–7.48], p=0.026 and HR 6.19 [95% CI 2.07–18.5], p<0.001, respectively) when compared to their references (IPI<2 and aaIPI<1, respectively). For the other indexes tested, no significant differences were found when compared with their reference groups. (Table 3 and figures 1A and 1B). aaIPI ≥ 2 was the only risk factor for a lower OS (HR 7.39 [95% CI 1.64–32.22], p = 0.002, when compared to its reference (aaIPI <2). For the other indexes tested, no significant differences were found when compared with their reference groups (Table 3, Figure 1D). Each variable included in the evaluated indexes was tested in a univariate analysis for PFS and OS. In this analysis, LDH was the variable with the highest significance for PFS (Table 4).
Table 3.
Univariate and multivariate analysis for outcomes
| Univariate analysis for PFS and OS | ||||
|---|---|---|---|---|
| Progression Free Survival | Overall survival | |||
| Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | |
| IPI - 0–2 - 3–5 |
1 2.86 (1.09–7.48) |
0.026 |
1 2 (0.65–6.14) |
0.219 |
| aaIPI - 0–1 - 2–3 |
1 6.19 (2.07–18.5) |
0.001 |
1 7.39 (1.64–33.22) |
0.002 |
| HCT-CI - 0–2 - ≥3 |
1 0.63 (0.14–2.86) |
0.544 |
1 1.58 (0.26–9.53) |
0.614 |
| CCI - 0 - 1 - ≥2 |
1 0.79 (0.29–2.19) 1.35 (0.55–3.32) |
0.648 |
1 1.2 (0.37–3.89) 2.23 (0.78–6.36) |
0.298 |
| Multivariate analysis for PFS and OS | ||||
| Progression Free Survival | Overall survival | |||
| Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | |
| aaIPI - 0–1 - 2–3 |
1 6.76 (2.21–20.69) |
0.001 |
1 7.91 (1.74–35.85) |
0.007 |
IPI: International Prognostic Index, aaIPI: Age adjusted International Prognostic Index, HCT-CI: Hematopoietic Transplant Comorbidities Indes, CCI: Charlson Comorbidity Index
Figure 1. Survival Kaplan-Meier curves.
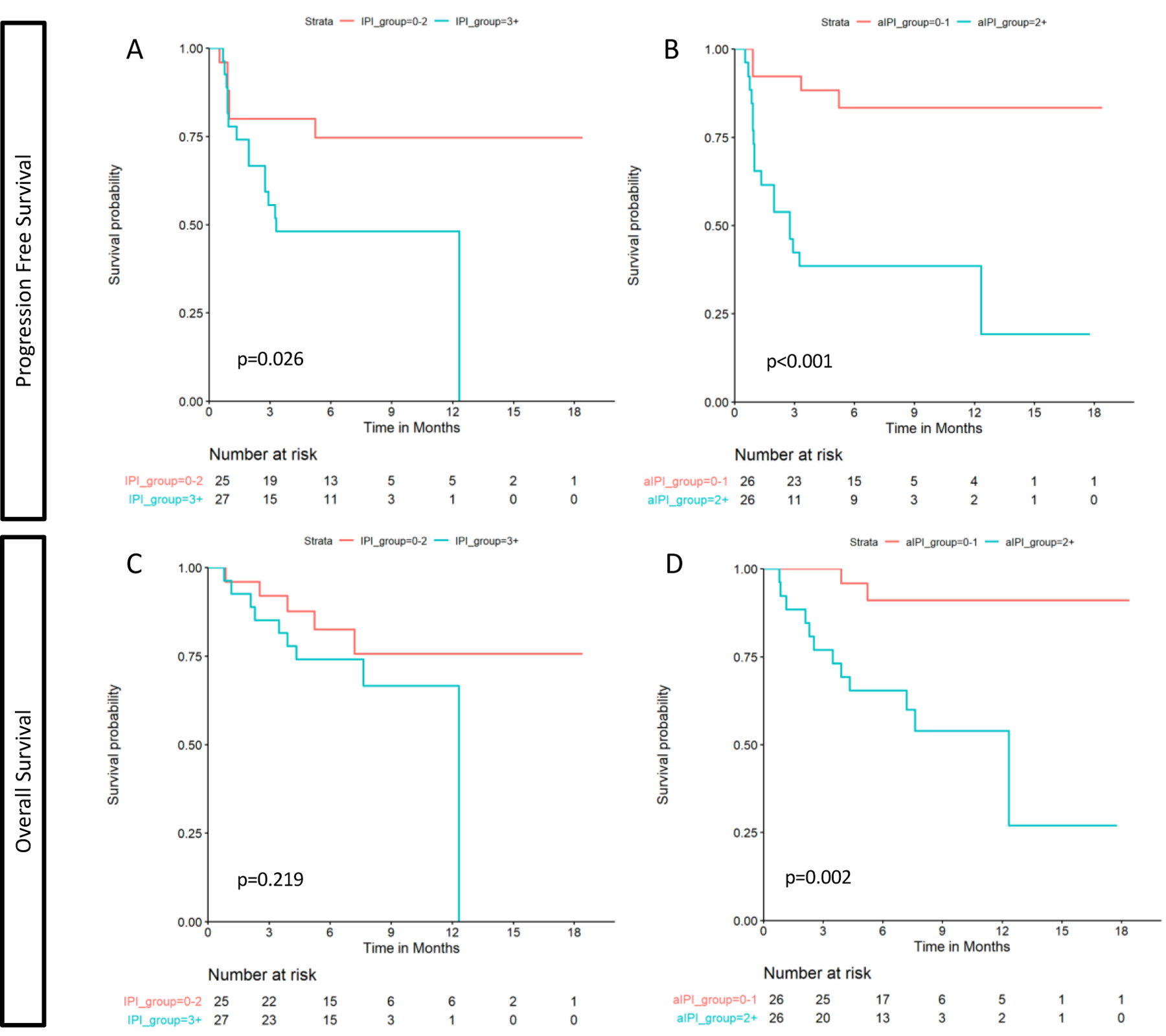
Progression free survival curves for IPI (A) and aaIPI (B). Overall survival curves for IPI (C) and aaIPI (D).
Table 4.
Univariate analysis of patient characteristics for outcomes
| Univariate analysis for PFS and OS | |||||||
|---|---|---|---|---|---|---|---|
| Progression Free Survival | Overall survival | ||||||
| Hazard Ratio (95%CI) |
P-value | Q-value | Hazard Ratio (95%CI) |
P-value | Q-value | ||
| Age >60 | 0.72 (0.34–1.55) | 0.4 | 0.8 | 0.52 (0.21–1.28) | 0.2 | 0.4 | |
| High LDH | 5.32 (2.13–13.3) | <0.001 | 0.008 | 6.55 (1.90–22.5) | 0.003 | 0.069 | |
| Stage III/IV | 3.85 (0.51–28.8) | 0.2 | 0.8 | - | - | - | |
| PS ≥ 1 | 1.67 (0.63–4.41) | 0.3 | 0.8 | 2.21 (0.73–6.74) | 0.2 | 0.4 | |
| NET > 1 | 1.93 (0.80–4.65) | 0.14 | 0.8 | 2.21 (0.74–6.60) | 0.2 | 0.5 | |
| Presence of arrhythmias | 0.57 (0.17–1.92) | 0.4 | 0.8 | 0.28 (0.04–2.10) | 0.2 | 0.5 | |
| Cardiac issues | 1.17 (0.4–3.38) | 0.8 | >0.9 | 1.39 (0.40–4.79) | 0.6 | 0.7 | |
| Diabetes | 1.17 (0.35–3.9) | 0.8 | >0.9 | 2.45 (0.69–8.67) | 0.2 | 0.4 | |
| Cerebrovascular disease | 1.61 (0.37–6.88) | 0.5 | 0.8 | 1.01 (0.13–7.64) | >0.9 | >0.9 | |
| Psychiatric disturbance | 1.38 (0.52–3.65) | 0.5 | 0.8 | 1.75 (0.58–5.34) | 0.3 | 0.5 | |
| Hepatic, mild | 2.49 (0.74–8.40) | 0.14 | 0.7 | 6.35 (1.71–23.6) | 0.006 | 0.07 | |
| Obesity | 0.67 (0.20–2.24) | 0.5 | 0.8 | 0.52 (0.12–2.33) | 0.4 | 0.6 | |
| Infection | 1.58 (0.64–3.92) | 0.3 | 0.8 | 2.12 (0.75–5.98) | 0.2 | 0.4 | |
| Rheumatologic | 1.07 (0.32–3.57) | >0.9 | >0.9 | 2.02 (0.58–6.99) | 0.3 | 0.5 | |
| Peptic ulcer | 2.08 (0.62–6.96) | 0.2 | 0.8 | 2.06 (0.47–9.10) | 0.3 | 0.5 | |
| Moderate/severe renal | 2.26 (0.30–16.8) | 0.4 | 0.8 | 1.87 (0.24–14.7) | 0.6 | 0.7 | |
| Prior solid tumor | 1.07 (0.32–3.56) | >0.9 | >0.9 | 0.46 (0.06–3.49) | 0.5 | 0.6 | |
| Heart valve disease | 0.89 (0.31–2.58) | 0.8 | >0.9 | 1.10 (0.32–3.80) | 0.9 | >0.9 | |
| Severe pulmonary | 1.73 (0.52–5.77) | 0.4 | 0.8 | 2.28 (0.65–8.01) | 0.2 | 0.5 | |
| Moderate/severe hepatic | 3.72 (0.87–16) | 0.07 | 0.6 | 3.62 (0.8–16.3) | 0.09 | 0.4 | |
| Variables only included in CCI | |||||||
| Myocardial infarct | 1.43 (0.49–4.13) | 0.5 | 0.8 | 1.67 (0.48–5.78) | 0.4 | 0.6 | |
| Chronic pulmonary disease | 0.57 (0.08–4.19) | 0.6 | 0.8 | 0.72 (0.09–5.52) | 0.8 | 0.8 | |
| Connective tissue disease | 1.07 (0.32–3.57) | >0.9 | >0.9 | 2.02 (0.58–6.99) | 0.3 | 0.5 | |
| Diabetes with end-organ damage | 0.93 (0.13–6.86) | >0.9 | >0.9 | 1.66 (0.22–12.5) | 0.6 | 0.7 | |
| Any Solid tumor last 5y | 0.69 (0.09–5.11) | 0.7 | >0.9 | - | - | - | |
Inflammatory bowel disease, moderate pulmonary impairment, congestive heart failure, peripheral vascular disease, dementia, hemiplegia, metastatic solid tumor and AIDS could not be evaluated due to the low incidence of cases in our series. Empty cells correspond to variables that do not converge for the model tested.
Since the aaIPI was identified as the index with the highest significance in the univariate analysis, multivariate models were performed with aaIPI for PFS and OS. In the multivariate analysis, aaIPI ≥ 2 was associated with inferior PFS (HR 6.76 [95% CI 2.21–20.69], p=0.001) and OS (HR 7.91 [95% CI 1.74–35.85], p=0.007) (Table 3).
CAR T cell-related toxicities and ICU admission
Forty-four (73.3%) patients developed CRS (n=44, 73.3%, median grade 2 [1–4]) or neurotoxicity (n=23, 38.3%, median grade 3 [1–4]) during the first 30 days after the CAR T cell infusion. Sixteen (26.7%) patients required admission to the intensive care unit (ICU). The median length of stay in the ICU was 5 days (1–12). All patients admitted to the ICU had CRS (median grade of 2 [2–4]) and 14 (87.5%) patients had neurotoxicity (median grade 3 [1–4]).
In the univariate analysis for any toxicity, HCT-CI ≥ 3 was associated with higher incidence of CRS or neurotoxicity (HR 3.53 [95% CI 1.24–10.05], p=0.018) (Table 5). For the other indexes tested, no significant differences were found when compared with their reference groups. In the univariate analysis for CRS, HCT-CI ≥ 3 was associated with higher incidence of CRS (HR 3.53 [95% CI 1.24–10.05], p = 0.018). Other indexes showed no significant differences when compared with their reference groups. The univariate analysis for neurotoxicity was significant for an association between IPI ≥ 3 and higher incidence of neurotoxicity (HR 2.53 [95% CI 1.01–6.38], p = 0.048) when compared with its reference group (IPI<2). Other indexes showed no significant differences when compared with their reference groups.
Table 5.
Univariate analysis for toxicities
| Univariate analysis for CRS1 and neurotoxicity combined | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CRS and NTX | CRS and NTX | ||||||||
| Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | ||||||
| IPI - 0–2 - 3–5 |
1 1.45 (0.83–2.53) |
0.186 |
1 1.69 (0.55–5.19) |
0.361 | |||||
| a-IPI - 0–1 - 2–3 |
1 1.1 (0.63–1.92) |
0.733 |
1 2.72 (0.79–9.37) |
0.113 | |||||
| HCT-CI - 0–2 - ≥3 |
1 3.53 (1.24–10.05) |
0.018 |
1 2.16 (0.43–10.75) |
0.347 | |||||
| CCI - 0 - 1 - ≥2 |
1 1.54 (0.83–2.86) 1.53 (0.77–3.05) |
0.27 |
1 2.11 (0.71–6.3) 0.71 (0.15–3.32) |
0.291 | |||||
| Univariate analysis for each toxicity | |||||||||
| CRS | CRS grade 3–4 | NTX | NTX grade 3–4 | ||||||
| Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | Hazard Ratio (95%CI) |
P-value | ||
| IPI - 0–2 - 3–5 |
1 1.22 (0.69–2.16) |
0.505 |
1 2.39 (0.47–12.09) |
0.291 |
1 2.53 (1.01–6.38) |
0.048 |
1 1.46 (0.46–4.65) |
0.525 | |
| a-IPI - 0–1 - 2–3 |
1 0.91 (0.51–1.63) |
0.754 |
1 5.91 (0.71–49.32) |
0.101 |
1 1.75 (0.75–4.09) |
0.197 |
1 2.42 (0.7–8.4) |
0.163 | |
| HCT-CI - 0–2 - ≥3 |
1 3.53 (1.24–10.05) |
0.018 |
1 0.53 (0.05–5.41) |
0.589 |
1 2.8 (0.58–13.6) |
0.201 |
1 4.2 (0.58–30.45) |
0.155 | |
| CCI - 0 - 1 - ≥2 |
1 1.27 (0.67–2.42) 1.3 (0.61–2.77) |
0.666 |
1 2.84 (0.61–13.24) 0.93 (0.09–9.23) |
0.357 |
1 2.1 (0.87–5.04) 0.99 (0.33–2.92) |
0.205 |
1 2.24 (0.74–6.82) 0.82 (0.17–4.06) |
0.275 | |
CRS: cytokine release syndrome; NTX: neurotoxicity. IPI: International Prognostic Index, aaIPI: Age adjusted International Prognostic Index, HCT-CI: Hematopoietic Transplant Comorbidities Indes, CCI: Charlson Comorbidity Index
No significant differences were found for any index tested when compared with their reference groups in the univariate analysis for severe CRS or severe neurotoxicity (Table 5).
DISCUSSION:
Prognostic indexes routinely used in patients with aggressive NHL such as IPI or aaIPI (15,23–25), or those commonly used in SCT recipients like the HCT-CI (20,26–29) are not routinely implemented in patients receiving commercial CD19 CAR T cell products. Other comorbidity indexes like the CCI (19) have not yet been studied in this setting either. In the present report, we demonstrate the association of IPI and aaIPI with PFS in patients with R/R DLBCL treated with commercial CAR T cell products. The aaIPI also is associated with OS, while the IPI is associated with neurotoxicity. Furthermore, in a subset of patients where it was evaluable, the HCT-CI was associated with early toxicity after CAR T cells infusion.
LDH is a risk factor included in both the IPI (age, tumor stage, LDH, performance status or PS and number of extranodal disease sites) and aaIPI (tumor stage, LDH and PS). In our study, of all indexes evaluated, aaIPI has the strongest association with PFS and OS (p = 0.001 and p = 0.002, respectively). This fact can be explained by the prominent weight of LDH in this index over the others. Different groups have identified LDH as a relevant prognostic factor in patients with R/R NHL treated with CAR T cell products. A recent analysis of patients’ baseline characteristics and their association with treatment efficacy in the Juliet trial showed that high levels of pre-infusion LDH, pre-infusion grade 3–4 thrombocytopenia, and grade 3–4 neurotoxicity were associated with inferior outcomes (PFS and OS)(30). In a comparative study of patients with R/R NHL treated with autologous stem cell transplantation (ASCT) vs. CD19 CAR T cell therapy (31), the only independent unfavorable factor for both PFS and OS was an elevated LDH level (p = 0.012 and p = 0.005, respectively). Interestingly, other risk factors like age, disease stage, or IPI ≥ 3 had no statistical significance in the multivariate analysis for OS in this study. However, the sample of patients included was small, and cases included had different histologies with different prognosis. In our series, the majority of patients had a good PS with advanced disease stage, reinforcing the role of LDH as outcome predictor.
Recently, Hirayama et al (32) also reported the association between an elevated pre-lymphodepletion LDH level and poor outcomes (PFS) in patients with R/R aggressive NHL receiving CD19 CAR T cells. Furthermore, this group identified a favorable cytokine profile at day 0 (defined as elevated monocyte chemoattractant protein-1, MCP-1, and IL-7) that could overcome the negative impact of an elevated LDH on PFS. An intense lymphodepletion regimen was associated with a higher probability of presenting a favorable cytokine profile. Other biomarkers that have been identified to impact outcomes are CRP < 30 mg/L and peak ferritin < 5.000 μg/L, both associated with improved PFS and OS(33).
PS ≥ 2, elevated LDH, soft tissue involvement or high metabolic tumor volume (MTV) are variables negatively associated with outcomes after CAR T cells (34–36). The impact of age, however, remains controversial. While a subanalysis of the 2-year follow-up of the ZUMA-1 trial showed survival rates are unaffected by age (37), a real-life study showed a negative tendency in the outcomes of young patients (34). This controversy supports our results favoring aaIPI over IPI as an outcome predictor, which also contrasts with the original publication of the scores (15). We cannot however exclude selection bias, particularly when it comes to older patients treated with these potentially toxic therapies.
Regarding early CD19 CAR-T cell therapy toxicities, we now show an association between high risk IPI and the development of neurotoxicity in patients with DLBCL treated with CD19 CAR T cells. In line with our results, other groups have studied IPI in lymphoma patients treated with CD19 CAR T cells and found no association between the IPI risk groups and the incidence of CRS (38). As the development of early toxicities seems to be related with the in vivo CAR T cell expansion after antigen recognition (39–41), it is not surprising that tumor burden, the intensity of lymphodepletion chemotherapy and CAR T cell dose have been identified as risk factors for CRS and neurotoxicity (42,43). Other factors associated with toxicity include thrombocytopenia before lymphodepletion for CRS (44), and pre-existing neurological comorbidity for neurotoxicity (45). Both severe CRS and neurotoxicity have also been associated with high levels of IL-6, IFN-γ, IL-15 and ferritin at baseline, before lymphodepletion (46,47). The effect of age on toxicity has also been analyzed. A recent real-world analysis based on the FDA Adverse Events Reporting System (FAERS) database of commercial CAR T cells a higher incidence of neurotoxicity (50% vs 39%, p = 0.04) in patients over 65, while younger patients (<65) had a higher incidence of CRS (80% vs 67%, p<0.01) (48). A prognostic model of neurotoxicity after axi-cel that combined clinical and laboratory factors, age was significantly associated with the development of neurotoxicity (49).
Finally, we also studied two well-known indexes in the transplant setting: the CCI and HCT-CI. While the CCI is a comorbidity index used to predict mortality in different malignancies (19), it includes comorbidities rarely present in SCT patients and also has limitations capturing comorbidities frequently present in these patients. Thus, in 2005 CCI was adapted to the transplant setting with the development of the HCT-CI (20). The HCT-CI is a helpful tool for selecting patients for SCT as it predicts NRM and survival after transplant. In order to calculate the HCT-CI, however, baseline testing needs to include PFTs and an echocardiogram, tests that are not typically required before a CAR T cell infusion. Consequently, only 15 of the 60 patients included were evaluable for the HCT-CI. Despite HCT-CI being associated with higher incidence of early toxicity after treatment in our study, we cannot draw definitive conclusions due to the small sample size. In addition, the patients who had PFTs and an echocardiogram performed may have represented a sicker group of patients as they had higher incidence of comorbidities than the full cohort. Furthermore, other groups tested HCT-CI in larger cohorts without finding a correlation with toxicity or outcomes (50). As expected, considering the limitations of the score, we did not find any association between CCI and the incidence of toxicities or outcomes.
Limitations of this study include the retrospective nature of the data collection and the small sample size available to evaluate HCT-CI.
In conclusion, we demonstrate a relationship between the IPI and aaIPI and PFS in patients with R/R DLBCL treated with commercial CD19 CAR T cells, demonstrating the relevance of these indexes as prognostic models in this patient population. Furthermore, we found that aaIPI is also associated with OS and IPI with the development of neurotoxicity in these patients. IPI and aaIPI are two simple and widely used tools in the management of aggressive NHL patients that can now provide better information to predict who would best benefit from CD19 CAR T cell therapy.
HIGHLIGHTS.
High-risk IPI and aaIPI are associated with poor PFS in R/R DLBCL after CAR T cells
High-risk aaIPI is associated with inferior OS in R/R DLBCL after CAR T cells
High-risk IPI is associated with neurotoxicity in R/R DLBCL after CAR T cells
ACKNOWLEDGEMENTS:
This work was supported by National Institutes of Health (NIH), National Cancer Institute Cancer Center Support Grant P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. K.W. received salary support from Parker Institute of Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center. M.P. was supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship and by Associazione italiana contro le leucemie-linfomi e mieloma Milano e Provincia ONLUS.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST:
M.G.R. received honoraria from Janssen and Takeda. K.W., M.P., R.A.T., J.F., R.S., A.O.A, M.L.S., S.D., E.H., J.R., M.M. and E.M. have no relevant conflict of interest. P.D. has served as a paid consultant on advisory boards for Kite. C.W.B. has received research support from Janssen, Novartis, Epizyme, Xynomics and Bayer. She has served as a paid consultant on advisory boards for Life Sci, GLG, Juno/Celgene, Seattle Genetics and Kite. She received honoraria from Dava Oncology. S.G. has received consultant fees and honoraria related to speakers’ bureau activities from Celgene Corporation, Genzyme Corporation, and Millennium Pharmaceuticals, Inc. L.P. is on the advisory committee of Pharmacyclics and provide consultancy for Noble insights. B.S. has consulted for Kite/Gilead, Juno/Celgene and Novartis. C.S.S. has served as a paid consultant on advisory boards for: Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene, Gamida Cell and GSK. He has received research funds for clinical trials from: Juno Therapeutics, Celgene, Precision Biosciences and Sanofi-Genzyme. M.S. is a consultant for McKinsey & Company and Angiocrine Bioscience Inc. S.G. received research support from Amgen and Janssen. M.A.P. reports honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite/Gilead and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of American Society for Transplantation and Cellular Therapy (ASTCT) and Be the Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Committee.
REFERENCES:
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edition (IARC WHO Classification of Tumours, Volume 2) [Internet]. International agency for research on Cancer (IARC). 2017. 4002 p. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:WHO+Classification+of+Tumours+of+Haematopoietic+and+Lymphoid+Tissues+,+Fourth+Edition#5 [Google Scholar]
- 2.Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 2019;94(5):604–16. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, Plantier I, Castaigne S, Lefort S, et al. Long-term outcome of patients in the LNH-98 . 5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients : a study by the Groupe d ‘ Etudes des Lymphomes de l ‘ Adulte. Blood. 2010;116(12):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, Neste E Van Den, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, Lejajis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, Moerloose B De, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, Mcguirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro A, Bezerra E, Hirayama A, Hill J, Wu V, Voutsinas J, et al. Late events after treatment with CD19-Targeted Chimeric Antigen Receptor Modified T-cells. Biol blood marrow Transplant. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigor EJM, Fergusson D, Kekre N, Atkins H, Seftel M, Daugaard M, et al. Risks and Benefits ofChimeric Antigen Receptor T-Cell (CAR-T) Therapy in Cancer: A Systematic Review and Meta-Analysis. Transfus Med Rev [Internet]. 2019;33(2):98–110. Available from: 10.1016/j.tmrv.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 10.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Shpall EJ. Chimeric antigen receptor T ‑ cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro-vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(56):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lulla PD, Hill LC, Ramos CA, Heslop HE. The Use of Chimeric Antigen Receptor T Cells in Patients With Non-Hodgkin Lymphoma. CLin Adv Hematol Oncol 2018;16(5):375–86. [PMC free article] [PubMed] [Google Scholar]
- 13.Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, et al. Comparing CAR T-cell toxicity grading systems: Application of the ASTCT grading system and implications for management. Blood Adv 2020;4(4):676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Young KH, Edeiros LJEM. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. [DOI] [PubMed] [Google Scholar]
- 15.Shipp MA, Harrington DP, Anderson JR. A predictive model for aggressive Non-Hodgkin’s Lymphoma. N Engl J Med 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 16.Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102(6):1989–96. [DOI] [PubMed] [Google Scholar]
- 17.Perales MA, Jenq R, Goldberg JD, Wilton AS, Lee SSE, Castro-Malaspina HR, et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45(9):1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation : Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant [Internet]. 2015;21(11):1863–9. Available from: 10.1016/j.bbmt.2015.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation ( HCT )– specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moskowitz CH, Nimer SD, Glassman JR, Portlock CS, Yahalom J, Straus DJ, et al. The International Prognostic Index predicts for outcome following autologous stem cell transplantation in patients with relapsed and primary refractory intermediate-grade lymphoma. Bone Marrow Transplant. 1999;23(6):561–7. [DOI] [PubMed] [Google Scholar]
- 22.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–38. [DOI] [PubMed] [Google Scholar]
- 23.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. [DOI] [PubMed] [Google Scholar]
- 24.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International Prognostic Index Remains a Valid Predictor of Outcome for Patients With Aggressive CD20 + B-Cell Lymphoma in the Rituximab Era. J Clin Oncol 2010;28(14):2373–80. [DOI] [PubMed] [Google Scholar]
- 25.Lerner RE, Thomas W, Defor TE, Weisdorf DJ, Burns LJ. The International Prognostic Index Assessed at Relapse Predicts Outcomes of Autologous Transplantation for Diffuse Large-Cell Non-Hodgkin’s Lymphoma in Second Complete. Biol Blood Marrow Transplant. 2007;13(4):486–92. [DOI] [PubMed] [Google Scholar]
- 26.Barba P, Ratan R, Cho C, Ceberio I, Hilden P, Devlin SM, et al. The hematopoietic cell transplantation comorbidity index (HCT- CI) predicts outcomes in patients with acute myeloid leukemia and myelodysplastic syndromes receiving CD34+ selected grafts for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veeraputhiran M, Yang L, Sundaram V, Arai S, Lowsky R, Miklos D, et al. Validation of the Hematopoietic Cell Transplantation – Specific Comorbidity Index in Nonmyeloablative Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant [Internet]. 2017;23(10):1744–8. Available from: 10.1016/j.bbmt.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berro M, Arbelbide JA, Rivas MM, Basquiera AL, Ferini G, Vitriu A, et al. Hematopoietic Cell Transplantation – Specific Comorbidity Index Predicts Morbidity and Mortality in Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant [Internet]. 2017;23(10):1646–50. Available from: 10.1016/j.bbmt.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 29.Salit RB, Oliver DC, Delaney C, Sorror ML, Milano F. Prognostic Value of the Hematopoietic Cell Transplantation Comorbidity Index for Patients Undergoing Reduced-Intensity Conditioning Cord Blood Transplantation. Biol Blood Marrow Transplant [Internet]. 2017;23(4):654–8. Available from: 10.1016/j.bbmt.2017.01.084 [DOI] [PubMed] [Google Scholar]
- 30.Westin JR, Tam CS, Borchmann P, Jaeger U, McGuirk JP, Edmund HH, et al. Correlative Analyses of Patient and Clinical Characteristics Associated with Efficacy in Tisagenlecleucel- Treated Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients in the Juliet Trial. Abstr 4103, 61st ASH Annu Meet Expo 2019;29–31. [Google Scholar]
- 31.Li C, Zhang Y, Zhang C, Chen J, Lou X, Chen X, et al. Comparison of CAR-T19 and autologous stem cell transplantation for refractory / relapsed non-Hodgkin’s lymphoma. JCI insight. 2019;23(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirayama Gauthier, Hay Voutsinas, Wu Gooley, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson C, Hunter B, Redd R, Rodig S, Chen P-H, Wright K, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol 2020;JCO.19.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol 2020;JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Li P, Ye S, Tang X, Wang J, Liu J, et al. Different sites of extranodal involvement may affect the survival of patients with relapsed or refractory non-Hodgkin lymphoma after chimeric antigen receptor T cell therapy. Front Med [Internet]. 2020. August 13;Online ahead of print. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32789732 [DOI] [PubMed]
- 36.Dean EA, Mhaskar RS, Lu H, Mousa MS, Krivenko GS, Lazaryan A, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 2020;4(14):3268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. A Comparison of 2-Year Outcomes in ZUMA-1 (Axicabtagene Ciloleucel [Axi-Cel]) and SCHOLAR-1 in Patients (Pts) with Refractory Large B Cell Lymphoma (LBCL). Biol Blood Marrow Transplant [Internet]. 2020. March 1 [cited 2020 Feb 22];26(3):S232. Available from: https://www.sciencedirect.com/science/article/pii/S108387911931403X [Google Scholar]
- 38.Zhiling Y, Huanxin Z, Wang Y, Cao J, Qiao J, Li D, et al. Risk Factors and Characteristics of CRS in CAR-T Treatment. Abstr 4462, 61st ASH Annu Meet Expo 2019; [Google Scholar]
- 39.Turtle CJ. Chimeric antigen receptor modified T cell therapy for B cell malignancies. Int J Hematol 2014;99(2):132–40. [DOI] [PubMed] [Google Scholar]
- 40.Hay KA, Turtle CJ. Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs. 2017;77(3):237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov 2018;8(8):958–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol 2018;183(3):364–74. [DOI] [PubMed] [Google Scholar]
- 43.Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T therapy for hematologic malignancies. J Allergy Clin Immunol [Internet]. 2020. August 6;Online ahead of print. Available from: 10.1016/j.jaci.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 44.Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor – modified T-cell therapy. Immunobiol Immunother 2017;130(21):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gust J, Hay KA, Hanafi L, Li D, Myerson D, Gonzalez-cuyar LF, et al. Endothelial Activation and Blood – Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017;7(12):1404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain MD, Faramand R, Staedtke V, Bai R, Lee SB, Kotani H, et al. The Lymphoma Tumor Microenvironment Influences Toxicity after CD19 CAR T Cell Therapy. Abstr 4105, 61st ASH Annu Meet Expo 2019;10:2–3. [Google Scholar]
- 47.Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T-cell Therapy for Lymphoma: Predictive Biomarkers and Clinical Outcomes. Neuro Oncol [Internet]. 2020. August 5;Online ahead of print. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32777012 [DOI] [PMC free article] [PubMed]
- 48.Zettler ME, Feinberg BA, Jr. EGP, Klink AJ, Mehta S, Gajra A. Real-World Analysis of Adverse Events Associated with CAR T-Cell Therapy Among Adults Age ≥65 Years. Abstr 1951, 61st ASH Annu Meet Expo 2019;1–2. [Google Scholar]
- 49.Rubin DB, Al Jarrah A, Li K, LaRose S, Monk AD, Ali AB, et al. Clinical Predictors of Neurotoxicity After Chimeric Antigen Receptor T-Cell Therapy. JAMA Neurol [Internet]. 2020. August 10;Online ahead of print. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32777012 [DOI] [PMC free article] [PubMed]
- 50.Kittai AS, Gordon MJ, Mian A, Fitzgerald L, Bishop J, Stephens D, et al. Comorbidities Predict Inferior Survival in Patients Receiving CAR T-Cell Therapy for Relapsed/Refractory DLBCL: A Multicenter Retrospective Analysis. Abstr 780, 61st ASH Annu Meet Expo 2019; [Google Scholar]


