Abstract
Survivors of COVID-19 are a vulnerable population, with complex needs because of lingering symptoms and complications across multiple organ systems. Those who required hospitalization or intensive care are also at risk for post-hospital syndrome and post-ICU syndromes, with attendant cognitive, psychological, and physical impairments, and high levels of health care utilization. Effective ambulatory care for COVID-19 survivors requires coordination across multiple subspecialties, which can be burdensome if not well coordinated. With growing recognition of these needs, post-COVID-19 clinics are being created across the country. We describe the design and implementation of multidisciplinary post-COVID-19 clinics at two academic health systems, Johns Hopkins and the University of California-San Francisco. We highlight components of the model which should be replicated across sites, while acknowledging opportunities to tailor offerings to the local institutional context. Our goal is to provide a replicable framework for others to create these much-needed care delivery models for survivors of COVID-19.
Key Words: coronavirus, long COVID-19, outpatient care, post-COVID-19, post-ICU
Abbreviations: JH, Johns Hopkins; OPTIMAL, pOst-covid-19/PosT-Icu MultidisciplinAry cLinic; PACT, postacute COVID-19 team; PHS, post-hospitalization syndrome; PICS, post-intensive care syndrome; UCSF, University of California-San Francisco; ZSFG, Zuckerberg San Francisco General
Early in the coronavirus pandemic, approximately one in five patients with COVID-19 required hospitalization, and 5% required intensive care.1 Health systems have been tireless and creative in ramping up capacity to care for these acutely ill individuals; however, less attention has been given to the post-hospital care such patients will need.
Recognizing the increasing literature on the long-term health impacts of COVID-19, physicians in the Johns Hopkins (JH) and University of California-San Francisco (UCSF) hospital systems worked collaboratively to develop clinics for survivors of severe COVID-19. Building from the ground up, with no existing post-ICU clinic at either site, we considered how best to address the well-described cognitive, behavioral, and physical sequelae of post-hospitalization syndrome (PHS)2 and post-intensive care syndrome (PICS).3 We also noted that COVID-19 survivors would face additional challenges because of strict visitor restrictions, having often received prolonged sedation and mechanical ventilation. Moreover, COVID-19 can cause disabling chronic symptoms even in those with less severe acute disease.4, 5, 6, 7
An integrated multidisciplinary approach is imperative to (1) meet the immediate needs of this rapidly growing population of survivors, (2) follow survivors longitudinally to identify clinical patterns, and (3) integrate research efforts focused on understanding the complications and sequelae of COVID-19. Here, we detail the creation of new clinics for COVID-19 survivors at two academic medical centers, with attention to commonalities that are important to maintain across health systems, and to opportunities for tailoring COVID-19 recovery clinics to their local institutional context.
Illustrative Case
The patient is an 83-year-old Spanish-speaking woman recently discharged after hospitalization for COVID-19 pneumonia. She has type 2 diabetes mellitus, hypertension, and hearing loss. She lives with her daughter, who is her designated power of attorney. Her hospitalization was complicated by prolonged intubation in the ICU, delirium, stroke, and dysphagia. She was discharged to subacute rehabilitation at a local skilled nursing facility. She was later discharged home breathing room air; however, she remains dyspneic, anxious, and socially isolated. Her local hospital does not have a post-COVID-19 or post-ICU clinic, and her daughter asks her primary care physician about how and where to seek specialized care for her complex recovery process.
Rapid Design and Implementation of Post-COVID-19 Care Clinics
At the UCSF and JH University, we aimed to optimize the recovery of COVID-19 survivors by the following: (1) creation of a centralized resource, (2) streamlining access to multiple specialties, (3) standardizing assessments and care, and (4) iterative adaptation based on accumulating knowledge. Importantly, we aimed not to replace primary care services, but rather to support primary care physicians.
The JH postacute COVID-19 team (PACT) clinic and UCSF pOst-covid-19/PosT-Icu MultidisciplinAry cLinic (OPTIMAL) saw their first patients in April and June 2020, respectively. To date, JH PACT has seen 365 patients, UCSF OPTIMAL has seen 223 patients, and Zuckerberg San Francisco General (ZSFG) OPTIMAL (the main hospital for the County of San Francisco) has seen 45 patients. UCSF is currently tracking readmission rates, patient satisfaction data including scores of how well physicians communicate, and cost metrics. Design and structure specific to the JH PACT has been published elsewhere.8
How to Approach Starting a Post-COVID-19 Clinic
Here, we outline a structured seven-step approach to starting a post-COVID-19 clinic at your own institution, based on our experience at our institutions.
Consider Adapting a PICS Framework and Its Associated Metrics
Given the proportion of COVID-19 survivors requiring hospitalization, including in the ICU, we used a systematic PHS/PICS framework to structure the clinic model. This involves evaluating a patient’s physical function, pulmonary function, cognitive function, and mental health. The JH PACT constructed an electronic medical record template, anchored in the key PHS/PICS domains of physical function, cognition, and mental health, and pulmonary health, enabling physicians to systematically evaluate patients and document findings. The framework and template were adapted and successfully adopted by the OPTIMAL at UCSF and ZSFG (e-Appendix 1).
Clinics supporting populations at risk for PHS/PICS are typically multidisciplinary clinics, providing access to multiple disciplines for holistic care of patients recovering from hospitalization.9 However, these clinics have often been plagued by barriers to attending clinic,10 resulting in high no-show rates.11 Telehealth, which rapidly expanded during the COVID-19 pandemic, enabled our clinics to transcend barriers including transportation and physical space allocation.
Notably, the American Thoracic Society/European Respiratory Society coordinated International Task Force12 did not make a recommendation regarding routine referral to a PICS clinic after COVID-19 because they did not want to reduce equipoise in applying the guidelines to settings with limited resources. We also acknowledge that many patients who are post-COVID-19 have not been hospitalized. However, our collective experience has shown that the comprehensive multidisciplinary structure of the PICS clinic can still greatly benefit patients with persistent symptoms in multiple organ systems requiring multiple subspecialists, regardless of initial degree of illness. It is our goal in sharing our experiences to make this type of coordinated subspecialty care more broadly accessible.
We advise new clinics to make the PICS framework patient-centered by asking patients which parts of their recovery are most meaningful to them. Decide in advance which patient outcome measurements and quality metrics you want to collect over time, who will administer the measurement tools, and how they will be administered (virtual, in-person, or a combination). All of our sites are in the process of collecting patient-reported outcomes, including measures of dyspnea, physical function, cognitive function, and mental health. Using the well-validated core outcome measures for clinical research in acute respiratory failure survivors13 , 14 (eg, those available at improvelto.com/coms/) is strongly recommended to standardize assessments across institutions. However, although standardized outcomes are ideal, we acknowledge that different institutions’ metrics may vary according to what instruments local sites prefer and can pay for. Moreover, sites vary on how frequently to order pulmonary function tests and follow-up imaging given the lack of clarity from national guidelines. Also, given that different clinics may use different proportions of virtual vs in-person visits, there should be built-in flexibility and structured training to adapt assessments across both contexts.
Given limited guidelines regarding the ideal timing of imaging and physiological testing in COVID-19 survivors, our clinics have not routinely instituted standardized assessments (ie, tests routinely ordered based on timing); instead, such evaluations are based on individual patient needs assessments.
Decide on Team Structure and Form a Nimble Coalition
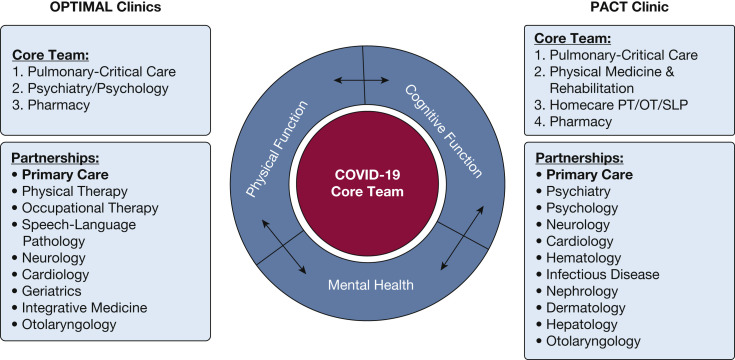
The pulmonary/critical care teams at both JH and UCSF partnered with multidisciplinary teams capable of supporting a PICS/PHS care framework. From inception, establishment of and communication with primary care was a central priority. Although these teams provide common examples, each institution’s team can be customized depending on local resources, expertise, and patient population needs. Figure 1 compares construction at each site. The Core Team members saw every single patient, whereas the Partnerships were streamlined consultative pathways that were available depending on the patients’ needs.
Figure 1.
Organizational structure of PACT and OPTIMAL post-COVID-19 clinics. OPTIMAL = pOst-covid-19/PosT-Icu MultidisciplinAry cLinic; PACT = postacute COVID-19 team; PT/OT/SLP = Physical Therapy/Occupational Therapy/Speech and Language Pathology.
To start a post-COVID-19 clinic at your institution, meet with key stakeholders to establish your coalition and team. Decide who will be the primary home for these patients—at some institutions, it will be pulmonary, at others, it might be primary care vs another subspecialty. Although post-COVID-19 clinics that follow a PICS framework can have high value when championed by pulmonary/critical care physicians who see patients in the ICU and in the outpatient setting, this is certainly not required. In fact, given the scalability of these models, parallel processes may be necessary to have pulmonary/critical care physicians see the patients who are post-COVID-19/post-ICU with separate capacity built within primary care to nonhospitalized patients with COVID-19; this division of labor may evolve over time as the pandemic surges continue to change.
Similarly, different team members may be in-person vs virtual, or seeing patients at the same visit or asynchronously, depending on clinic structure. Ideally, the patient would be able to have a one-stop-shop to see all relevant team members and subspecialists in the same day and visit, particularly given patients’ fatigue and activity limitations. We acknowledge that this level of logistics may not be possible, and our experience suggests that high-quality care can still be delivered asynchronously, with having as many key team members seeing the patient together as possible and the rest of the care delivered at other visits that are proximal in time. This asynchronous care model can be particularly beneficial in patients with significant fatigue and postexertional symptoms.
We have found that, for commonly encountered problems (eg, fatigue, brain fog, palpitations, other symptoms), the approach for each patient should be individualized. Some patients will benefit from primary consultation alone, whereas others with both brain fog and memory impairment and cognitive impairment can be referred to neurology and physical and occupational therapy. Patients with dysphagia can be referred to otolaryngology and speech and language pathology. As structured partnerships with other specialists and team members are developed, we recommend establishing firm criteria for mandatory vs elective referrals and testing required in advance. For example, a partnering cardiologist may want patients with palpitations to receive an echocardiogram prior to being seen; these workflows may differ from that of a typical post-ICU clinic.
The organization requires flexibility—the ability to scale up and down as needed, recognizing that the inpatient surges will be slower to dissolve and more enduring in the ambulatory settings. Teams will need to adapt and have the support and endurance to continue to care for this complex patient population, all while grappling with additional clinical responsibilities and responsibilities outside of work. Care must be taken to build sustainable team structures to avoid having clinic responsibilities be considered additional responsibilities.
Decide on Your Inclusion Criteria and Communication Strategy
The JH PACT and OPTIMAL focused on hospitalized patients (ICU and/or ward), while also providing a referral pathway for any COVID-19 survivor with persistent pulmonary and/or rehabilitation needs, regardless of initial disease severity. The prespecified target population and inclusion criteria (eg, postacute and/or community-dwelling patients) for emerging COVID-19 survivorship clinics will largely depend on the immediate priorities and available resources of the sponsoring institution and the prevalence of COVID-19 in the local community. Initially, we aimed to prospectively see patients at approximately 1, 3, 6, and 9 months after discharge. However, as the pandemic evolved, we departed from this standard approach and made individualized decisions about follow-up frequency and discharged patients to primary care based on their progress.
To encourage referrals, we educated key stakeholders, including ICU physicians, hospitalist and resident teams, and case management, regarding referral workflows (e-Appendix 2). Specifically, we wanted to lower the barriers to referral and therefore communicated with inpatient ICU and hospital physicians, case managers, and discharge coordinators, and primary care providers, urgent care providers, and emergency medicine providers, about referral pathways. Stakeholders were educated and engaged in several ways, including via communication with network directors, e-mails, PowerPoint (Microsoft) presentations, virtual grand round attendance, media/website presence, and other forms of outreach. From inception, communication with primary care or, for those patients who did not already have a primary care provider, establishment of primary care, was a central priority. At all sites, referrals to the clinic were incorporated into discharge checklists by hospitalist physicians and case management. Outpatient clinic referral coordinators played an essential role in educating and interfacing with inpatient teams, gathering relevant patient information, confirming referral qualifications, coordinating visit timing, and refining the referral process.
Champion Resource Allocation to Achieve Health Equity
Uninsured or underinsured patients—who may be at greater risk for COVID-19 complications15—often have difficulties navigating the health care system, which can further exacerbate vulnerabilities and disparities postdischarge. By providing a centralized resource after COVID-19-related hospitalization, the clinics helped patients navigate ambulatory pathways and establish or reconnect with primary care. To ensure equity and access, we used telehealth to transcend many potential barriers, including ability/functional status, lack of a prior connection with the health care system, and transportation resources.16 However, we anticipated that telemedicine could also be a barrier and exacerbate inequities; therefore, for those unable to navigate telemedicine, OPTIMAL has offered in-person or telephone visits. The ZSFG clinic was largely in-person given technology barriers and patient preference, whereas JH PACT and OPTIMAL were largely virtual for initial visits.
We paid special attention to make sure to systematically address patients’ social determinants of health and ask about employment status, social isolation, food security, and housing concerns. An embedded clinic social worker can be essential to address the complex social needs of patients and help connect patients to local resources. We provided standardized after-visit instructions that included links and information to these resources.
We acknowledge that both of our centers are housed in large, urban, academic medical centers. These structures can be adapted to a variety of contexts, with more creative use of virtual consultation to less accessible subspecialists, to improve access to high-quality care to more rural and lower-resource settings. Already, via use of telemedicine, we have been able to provide care to patients from rural communities with limited access to subspecialty care.
Advocate for Institutional Support
The bulk of described operations were initially embedded into existing clinical infrastructure, making them cost-neutral. However, our clinics are advocating for physical space alongside collaborating disciplines and funding to support collaborating staff, faculty, and clinic directors. When able, we recommend constructing a formal business plan prior to launch to highlight the financial sustainability of these clinics, including tracking referrals and follow-up testing, incorporating patients into primary care, optimizing return-to-work for employees, and improving readmission rates. High visit complexity is also an important consideration in institutional business modeling, particularly given increased use of time-based billing under new Center for Medicare Services Evaluation & Management guidelines.
Although these clinics may be a substantial financial investment, they could be financially sustainable in the future by providing referrals to other outpatient services and laboratory/imaging services and preventing readmissions and ED visits (Levan et al, unpublished data, April 2021). Moreover, coordinating the clinical enterprise with research efforts may also provide financial and brand synergy.
Negotiate with medical center leadership for salary support, protected clinical time, and staffing for positions including a medical director, clinic coordinator, and other critical staff from the multidisciplinary team. We recognize that it may be hard to successfully negotiate for protected time and support for all of these positions; however, if prioritization is needed, resources for pharmacy, physical therapy, psychiatry, clinic coordinators, and medical directors are among the highest-yield resources.
Coordinate With Research Efforts
The COVID-19 pandemic has created an unprecedented situation where institutions must meet the immediate needs of survivors while improving our understanding of potential long-term implications of acute illness. Ideally, research activities can synergize with clinical excellence. By creating standardized assessments and recording clinical information in a detailed and consistent way, clinical care can facilitate pattern recognition and directly inform future multi-institutional research efforts. Importantly, where possible, the electronic medical record should be leveraged to simplify efficient data abstraction. Clinics can also provide important populations for registries and clinical trial recruitment.
Rapid Cycle Iteration and Improvements
We communicated frequently with clinic teams about evolving workflow, literature, and telehealth guidelines. Consideration of infection control was a high priority for staff; therefore, clarifying safety precautions was essential. All clinic team members used droplet precautions with universal masking and eye-shield wearing; patients were not seen in-person until 4 weeks postdischarge, when they were thought to be no longer actively shedding virus and cleared by infection control. Additionally, we used scripted introductions by clinic referral coordinators and schedulers to educate patients on the role of the clinics and trained nurses to field commonly encountered patient questions and concerns. We created electronically housed and continually updated faculty development handbooks detailing literature updates, clinic workflow diagrams, and protocols. Based on feedback from patients, staff, and providers, we continually updated the faculty handbook, the electronic medical record template, and the clinic process and workflows. Residents, fellows, and interprofessional trainees were encouraged to join the clinical team as elective training experiences. Moreover, we implemented preclinic huddles, multidisciplinary meetings, and faculty conferences to discuss patient management and clinical innovations.
Regular multidisciplinary team meetings provide a venue to discuss individual patient needs and identify gaps in workflow and processes. Expect high demand once patients are aware of this service; inclusion criteria may have to be modified (either narrowed or broadened) depending on patient population prioritization, surge capacity, and available resources. Meet regularly with stakeholders to assess what is working well and improve inefficiencies. Disseminate and discuss the evolving literature on COVID-19 within the clinical team. Message your small wins and big wins at every step of the way. Open lines of communication with other centers, including national networks of post-ICU clinics such as the Critical and Acute Illness Recovery Organization,17 are important to share experiences and accelerate development and knowledge. Track outcomes upfront to better understand impact of the clinic and assist in justifying necessary expansion to health systems. Using a structured plan-do-study-act cycle for iterative process improvement may be helpful for a structured approach to repeated cycles of iteration, innovation, and improvement.18
Illustrative Case Concluded
The patient’s daughter referred her to a different institution in her area that had started a post-COVID-19 clinic. There, she received assessments of her dyspnea with pulmonary function tests and referral to pulmonary rehabilitation, instructions on breathing exercises, a detailed mental health evaluation for her anxiety, and deprescribing of nonessential medications by the team pharmacist. Ultimately, the patient told the team, “I feel like I understand my recovery process better and feel hope again.”
Conclusions
Rapid development and implementation of multidisciplinary post-COVID-19 clinics is feasible, even in systems with no existing infrastructure for post-ICU follow-up. Common barriers include securing protected time and space, establishing workflows, and coordinating with primary care. Rapid cycle iteration based on frequent meetings and key stakeholder input is essential to successful implementation and sustainability. Clinical excellence and research must be coordinated to simultaneously meet the immediate needs of survivors and better understand potential long-term consequences of COVID-19 infection.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Appendixes can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Brigham and Parker contributed equally to this manuscript.
Supplementary Data
References
- 1.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krumholz H.M. Post-hospital syndrome–a condition of generalized risk. N Engl J Med. 2013;368(2):100. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 4.Tenforde M.W., Kim S.S., Lindsell C.J., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelen M, Manoharan L, Elkheir N, et al. Characterising long-term Covid-19: a rapid living systematic review. medRxiv. 10.1101/2020.12.08.20246025. [DOI]
- 6.Venturelli S., Benatti S.V., Casati M., et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brigham E, O'Toole J, Kim SY, et al. The Johns Hopkins Post-Acute COVID-19 Team (PACT): a multidisciplinary, collaborative, ambulatory framework supporting COVID-19 survivors. Am J Med. 2021;134(4):462-467. [DOI] [PMC free article] [PubMed]
- 9.Sevin C.M., Bloom S.L., Jackson J.C., Wang L., Ely E.W., Stollings J.L. Comprehensive care of ICU survivors: development and implementation of an ICU recovery center. J Crit Care. 2018;46:141–148. doi: 10.1016/j.jcrc.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines K.J., McPeake J., Hibbert E., et al. Enablers and barriers to implementing ICU follow-up clinics and peer support groups following critical illness: the thrive collaboratives. Crit Care Med. 2019;47(9):1194–1200. doi: 10.1097/CCM.0000000000003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbertson B.H., Cuthbertson B.H., Rattray J., et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai C., Chotirmall S.H., Rello J., et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020) Eur Respir Rev. 2020;29(157):200287. doi: 10.1183/16000617.0287-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Needham D.M., Sepulveda K.A., Dinglas V.D., et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikkelsen M.E., Still M., Anderson B.J., et al. Society of Critical Care Medicine's international consensus conference on prediction and identification of long-term impairments after critical illness. Crit Care Med. 2020;48(11):1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 15.Gaffney A.W., Hawks L., Bor D.H., et al. 18.2 million individuals at increased risk of severe COVID-19 illness are un- or underinsured. J Gen Intern Med. 2020;35(8):2487–2489. doi: 10.1007/s11606-020-05899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur N., Lovinsky-Desir S., Bime C., Wisnivesky J.P., Celedón J.C. Health Equality and Diversity Committee of the American Thoracic Society. The structural and social determinants of the racial/ethnic disparities in the US COVID-19 pandemic: what's our role? Am J Respir Crit Care Med. 2020;202(7):943–949. doi: 10.1164/rccm.202005-1523PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CAIRO Critical and Acute Illness Recovery Organization. https://sites.google.com/umich.edu/cairo/home?authuser=0 Accessed December 1, 2020.
- 18.Taylor M.J., McNicholas C., Nicolay C., et al. BMJ Qual Saf. 2014;23(4):290–298. doi: 10.1136/bmjqs-2013-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.