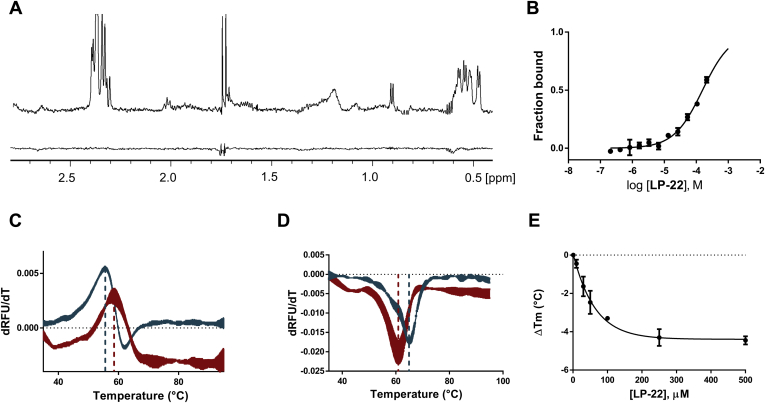
Figure 4.
Peptide LP-22 interacts at the LDH tetrameric interface, destabilizes tetrameric LDH, and stabilizes dimeric LDH.A, WaterLOGSY spectra of the interaction of LP-22 (400 μM) with dimeric LDH-Htr (up) and tetrameric LDH-1 (down) at 15 μM. B, MST binding curves between LP-22 and LDH-Htr. Binding curves were extracted from the MST traces at a 1.5 s MST on time (n = 3). C, nanoDSF denaturation of dimeric LDH-Htr (15 μM) with (red) and without (teal) LP-22 (500 μM) (ΔTm = 2.8 °C; n = 3). D, nanoDSF denaturation of tetrameric LDH-5 (300 nM) with (red) and without (teal) LP-22 (250 μM) (ΔTm = −4.3 °C; n = 3). E, ΔTm (°C) of tetrameric LDH-5 (300 nM) as a function of LP-22 concentration (EC50 = 47 μM [32–68 μM]; n = 3). LDH, lactate dehydrogenase; LDH-1, lactate dehydrogenase heart isozyme homotetramer; LDH-5, lactate dehydrogenase muscle isozyme homotetramer; LDH-Htr, LDH-H truncated; LP-22, cluster B1-derived peptide; MST, microscale thermophoresis; nanoDSF, nanoscale differential scanning fluorimetry; RFU, relative fluorescent unit; WaterLOGSY, water–ligand observed via gradient spectroscopy.