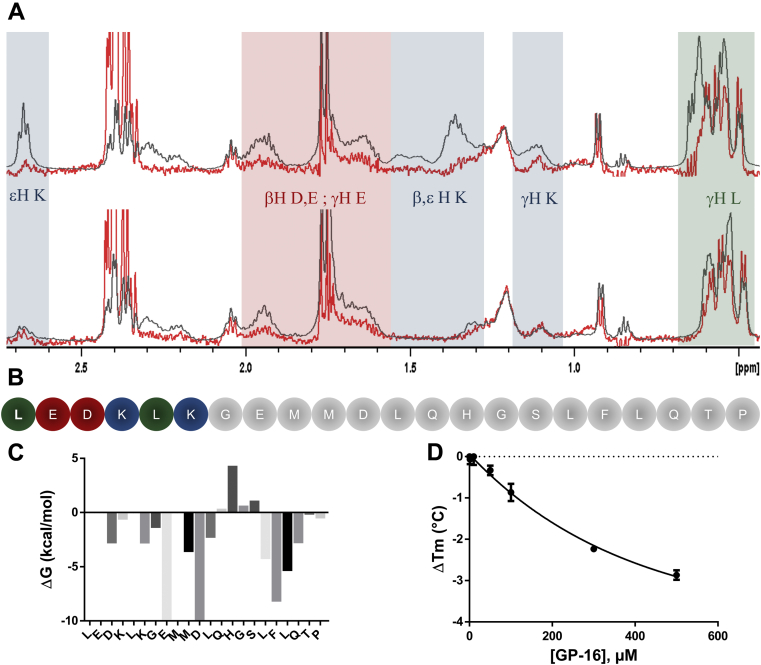
Figure 5.
LP-22 N-terminal trimming leads to GP-16 with a similar interaction profile.A, comparison of the difference between LP-22 (up) and GP-16 (bottom) WaterLOGSY (red) and 1H (black) NMR spectra in the presence of 15 μM of LDH-Htr. Signals that appear in the 1H spectra but not in WaterLOGSY correspond to noninteracting residues. LP-22 spectra highlight that some lysine (blue), glutamate (red), aspartate (red), and leucine (green) residues do not interact with LDH-Htr. These noninteracting signals are no longer present on the GP-16 spectra (down). B, peptide sequence of LP-22. Colored residues correspond to the residues that do not interact according to ΔG calculation and WaterLOGSY analysis. C, calculation of LP-22 residue contribution to the overall free energy of binding using the Molecular Operating Environment software. D, differences in melting temperature (ΔTm, °C) of tetrameric LDH-5 (300 nM) as a function of GP-16 concentration (EC50 = 262 μM [142–383 μM]; n = 3). LDH-5, lactate dehydrogenase muscle isozyme homotetramer; LDH-Htr, LDH-H truncated; LP-22, cluster B1-derived peptide; WaterLOGSY, water–ligand observed via gradient spectroscopy.