Abstract
Purpose
Although local control is an important issue for longer-term survivors of spinal metastases treated with conventional external beam radiation therapy (EBRT), the literature on radiographic local failure (LF) in these patients is sparse. To inform clinical decision-making, we evaluated rates, consequences, and predictors of radiographic LF in patients with spinal metastases managed with palliative conventional EBRT alone.
Methods and Materials
We retrospectively reviewed 296 patients with spinal metastases who received palliative EBRT at a single institution (2006-2013). Radiographic LF was defined as radiologic progression within the treatment field, with death considered a competing risk. Kaplan-Meier, cumulative incidence, and Cox regression analyses determined overall survival estimates, LF rates, and predictors of LF, respectively.
Results
There were 182 patients with follow-up computed tomography or magnetic resonance imaging; median overall survival for these patients was 7.7 months. Patients received a median of 30 Gy in 10 fractions to a median of 4 vertebral bodies. Overall, 74 of 182 patients (40.7%) experienced LF. The 6-, 12-, and 18-month LF rates were 26.5%, 33.1%, and 36.5%, respectively, while corresponding rates of death were 24.3%, 38.1%, and 45.9%. Median time to LF was 3.8 months. Of those with LF, 51.4% had new compression fractures, 39.2% were admitted for pain control, and 35.1% received reirradiation; median time from radiation therapy (RT) to each of these events was 3.0, 5.7, and 9.2 months, respectively. Independent predictors of LF included single-fraction RT (8 Gy) (hazard ratio [HR], 2.592; 95% confidence interval [CI], 1.437-4.675; P = .002), lung histology (HR, 3.568; 95% CI, 1.532-8.309; P = .003), and kidney histology (HR, 4.937; 95% CI, 1.529-15.935; P = .008).
Conclusions
Patients experienced a >30% rate of radiographic LF by 1 year after EBRT. Single-fraction RT and lung or kidney histology predicted LF. Given the high rates of LF for patients with favorable prognosis, assessing the risk of death versus LF is important for clinical decision-making.
Introduction
Approximately two-thirds of patients with cancer develop bony metastases during their lifetime, with the highest prevalence of bony metastases of 65% to 75% in patients with breast and prostate cancers.1, 2, 3 The most common site of bony metastasis is the spine.3 The main goals of treatment for spinal metastases are aimed at improving site-related symptoms, optimizing local control, and reducing risk of site-related spinal events, such as fracture and cord compression.4, 5, 6, 7, 8 Conventional external beam radiation therapy (EBRT) has traditionally been the primary modality of treatment for spinal metastases, with larger doses of radiation delivered in a single fraction or smaller doses over a multifractionated regimen.9,10 The treatment dose limitation in conventional EBRT is the toxicity of radiation to the spinal cord.11 Among patients with a more favorable prognosis or with radioresistant tumors, stereotactic body radiation therapy (SBRT) has emerged as a method for delivering high doses of radiation in a dose-escalated manner to the target vertebral region while minimizing radiation to nearby critical structures such as the spinal cord.9,12 For patients presenting with spinal cord compression, studies have also shown the benefits of radiation therapy (RT) combined with surgery in improving functional status.13, 14, 15, 16 In situations of spinal instability or vertebral collapse, surgery or vertebroplasty may restore spinal stability.17
Although current treatments may palliate symptoms or improve functional status, there are minimal data describing patterns of local failure (LF) in patients with spinal metastases treated with conventional EBRT alone, despite its relatively common occurrence. The few studies that have reported on LF for spinal metastases after treatment with conventional EBRT have focused on metastatic spinal cord compression and reirradiation as endpoints of LF, which may not sufficiently capture true radiographic LF events and their clinical sequelae.18, 19, 20, 21, 22 Given the clinical implications of recurrence in the spine, understanding patterns of LF after EBRT is critical. Our study aimed to comprehensively evaluate for rates, consequences, and predictors of radiographic LF within a cohort of patients treated with conventional EBRT alone for spinal metastases, to inform clinical decision-making for patients treated with this modality of RT.
Methods and Materials
Study population
After obtaining institutional review board approval, we performed a retrospective analysis of 296 consecutive patients with spinal metastases who were treated with EBRT alone at our institution between January 2006 and December 2013. For nearly all patients, EBRT was used to treat 1 vertebral body above, 1 vertebral body below, and the entire lateral extent of vertebral bodies with metastatic disease, as well as the set-up margin. There were 3 patients who had 1 to 2 vertebral bodies treated with RT. Each patient in this study had 1 radiation treatment site; treatment of vertebral bodies were continuous sites and not separate treatments. Patients who were treated with SBRT to the primary metastatic lesion and patients who had solely paraspinal, but not spinal, disease were excluded from the study.
Study parameters and endpoints
Patients’ medical records were reviewed for clinical characteristics. Metastatic burden, spine level, paraspinal disease, epidural involvement, cord compression, and radiologic progression noted on computed tomography (CT) or magnetic resonance imaging (MRI) were determined by the reviewing radiologist. Spinal instability was determined by a radiation oncologist according to the spinal instability neoplastic score (SINS) system, with a score of 1 to 6 denoting clinically stable lesions, 7 to 12 denoting potentially unstable lesions, and 13 to 18 indicating unstable lesions.23 Eastern Cooperative Oncology Group performance status, ambulatory status, and presence of neuropathic pain were assessed by the patient’s clinician.
The primary endpoints were rates of radiographic LF and time to development of radiographic LF, defined as time from the start of RT to radiologic progression within the treatment field of the irradiated metastasis, as determined by the reviewing radiologist and confirmed by a radiation oncologist. Secondary endpoints included: (1) time to consequences of LF, as manifested by new fractures, pain admissions, or neurologic symptoms and (2) time to interventions for LF, which included reirradiation of treated spinal metastasis, invasive interventions for pain control, salvage surgery, and vertebroplasty. Invasive interventions for pain control included local spinal injections, such as the placement of indwelling catheters and nerve ablation, at the site of radiographic LF.
Statistical analysis
Cumulative incidence estimates were used to determine radiographic LF rates, with death considered a competing risk. Censoring occurred at the date of last follow-up or date of death. We used X2 analyses to compare baseline characteristics between patients with versus without LF. We conducted Cox proportional hazards regression analyses to test predictors of LF and to calculate hazard ratios (HRs). Overall survival (OS) estimates were determined by the Kaplan-Meier method and compared using the log-rank test. Descriptive statistics were used to summarize estimates of time to consequences of LF and interventions for LF. Analyses were conducted with R version 3.5.2, with 2-sided tests and statistical significance set at 0.05.
Results
Patient characteristics
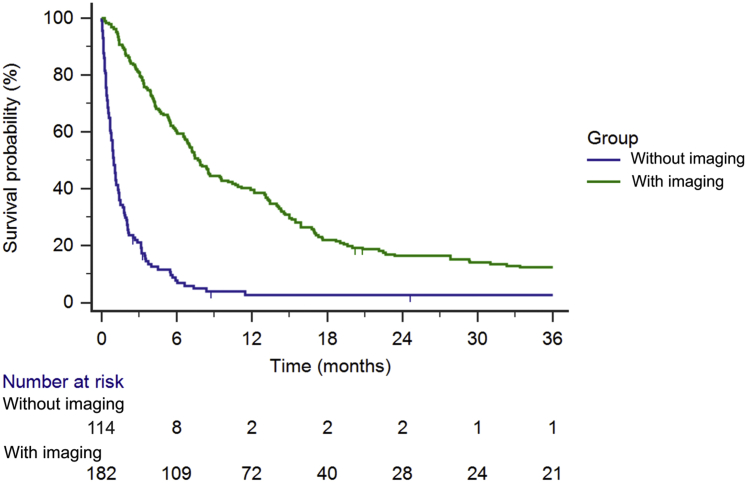
A total of 182 out of 296 patients who received conventional EBRT for spinal metastases had follow-up CT or MRI available for review. On average, patients received imaging every 3 to 4 months. Median OS for all 296 patients was 4 months. Of the 114 patients without imaging, median OS was 4 months; 99 patients (86.7%) had died within 4 months of RT (Fig 1). In contrast, median OS for patients with imaging was 7.7 months; 48 of the 182 patients (26.4%) with imaging had died within 4 months. Patients with compared to without imaging had statistically significant higher median OS (P < .0001) and represent a cohort with more favorable prognosis. Further analyses in this study include only the 182 patients who had received imaging, to allow for radiographic detection of LF.
Figure 1.
Overall survival in patients with spinal metastases managed with conventional radiation therapy with versus without follow-up imaging (N = 296).
Among the patients with imaging, median follow-up time was 7.9 months overall (range, 0-124 months) and 20 months for those surviving at least 1 year. The median age at the start of RT was 60.5 years (range, 23-97 years). Most patients (52.2%) received 30 Gy in 10 fractions to a median of 4 vertebral bodies. There were 32 patients (17.6%) who received a single fraction of 8 Gy. The most common primary tumor histology was lung (23.6%). The thoracic spine was the most common site of metastasis, occurring in 46.2% of patients. Just over half of patients (54.4%) had epidural involvement and 33.5% had paraspinal disease. Baseline clinical and radiation dose data for patients are summarized in Table 1.
Table 1.
Baseline clinical characteristics of patients with spinal metastases with radiologic imaging (n = 182)
| Overall (N = 182) |
Patients with local failure (n = 74) |
Patients without local failure (n = 108) |
Comparison of baseline characteristics (with vs without local failure) |
||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | P value | |
| Age at RT start (years) | |||||||
| Median | 60.5 | - | 59.5 | - | 61 | - | - |
| Range | 23-97 | - | 38-97 | - | 23-87 | - | - |
| Sex | |||||||
| Female | 100 | 54.9 | 35 | 47.3 | 65 | 60.2 | .086 |
| Male | 82 | 45.1 | 39 | 52.7 | 43 | 39.8 | |
| Time from metastatic diagnosis to RT start (years) | |||||||
| Median | 0.7 | - | 0.9 | - | 0.5 | - | - |
| Range | 0-12.4 | - | 0-9.1 | - | 0-12.4 | - | - |
| ECOG PS | |||||||
| 0 | 35 | 19.2 | 16 | 21.6 | 19 | 17.6 | .835 |
| 1 | 97 | 53.3 | 37 | 50 | 60 | 55.6 | |
| 2 | 34 | 18.7 | 15 | 20.3 | 19 | 17.6 | |
| 3 | 16 | 8.8 | 6 | 8.1 | 10 | 9.3 | |
| Histology | |||||||
| Breast | 40 | 22.0 | 9 | 12.2 | 31 | 28.7 | .013 |
| Lung | 43 | 23.6 | 17 | 23.0 | 26 | 24.1 | |
| Prostate | 32 | 17.6 | 17 | 23.0 | 15 | 13.9 | |
| Kidney | 10 | 5.5 | 8 | 10.8 | 2 | 1.8 | |
| Melanoma | 7 | 3.8 | 2 | 2.7 | 5 | 4.6 | |
| Other∗ | 50 | 27.5 | 21 | 28.4 | 29 | 26.9 | |
| Metastatic burden† | |||||||
| 1 | 41 | 22.5 | 18 | 24.3 | 23 | 21.3 | .497 |
| 2 | 58 | 31.9 | 24 | 32.4 | 34 | 31.5 | |
| 3 | 42 | 23.1 | 13 | 17.6 | 29 | 26.9 | |
| ≥4 | 41 | 22.5 | 19 | 25.7 | 22 | 20.4 | |
| Spine level | |||||||
| Cervical | 8 | 4.4 | 4 | 5.4 | 4 | 3.7 | .559 |
| Thoracic | 84 | 46.2 | 30 | 40.5 | 54 | 50.0 | |
| Lumbar | 74 | 40.7 | 34 | 45.9 | 40 | 37.0 | |
| Spine | 16 | 8.8 | 6 | 8.1 | 10 | 9.3 | |
| SINS | |||||||
| 0-6 | 71 | 39.0 | 28 | 37.8 | 43 | 39.8 | .786 |
| 7-12 | 99 | 54.4 | 40 | 54.1 | 59 | 54.6 | |
| ≥13 | 12 | 6.6 | 6 | 8.1 | 6 | 5.6 | |
| Ambulatory | 172 | 94.5 | 69 | 93.2 | 103 | 95.4 | .536 |
| Cord compression | 19 | 10.4 | 8 | 10.8 | 11 | 10.2 | .892 |
| Neuropathic pain‡ | 96 | 52.7 | 43 | 58.1 | 53 | 49.5 | .230 |
| Paraspinal disease‡ | 61 | 33.5 | 21 | 28.4 | 40 | 37.4 | .224 |
| Epidural involvement§ | 99 | 54.4 | 43 | 68.3 | 56 | 59.6 | .405 |
| Total RT dose (Gy) | |||||||
| Median | 30 | - | 30 | - | 30 | - | - |
| Range | 8-40 | - | 8-40 | - | 8-37.5 | - | - |
| Dose regimens | |||||||
| Single-fraction (8 Gy) | 32 | 17.6 | 18 | 24.3 | 14 | 13.0 | .048 |
| Multifraction‖ | 150 | 82.4 | 56 | 75.7 | 94 | 87.0 | |
| Cumulative BED¶ | |||||||
| 40 Gy | 32 | 17.6 | 18 | 24.3 | 14 | 13 | .197 |
| 41-60 Gy | 29 | 15.9 | 11 | 14.9 | 18 | 16.7 | |
| 61-75 Gy | 94 | 51.6 | 33 | 44.6 | 61 | 56.5 | |
| ≥76 Gy | 27 | 14.8 | 12 | 16.2 | 15 | 13.9 | |
| Number of vertebral bodies covered by RT | |||||||
| 1 | 2 | 1.1 | 0 | 0 | 2 | 1.9 | .525 |
| 2 | 1 | 0.5 | 0 | 0 | 1 | 0.9 | |
| 3 | 43 | 23.6 | 20 | 27 | 23 | 21.3 | |
| 4 | 50 | 27.5 | 20 | 27 | 30 | 27.8 | |
| 5 | 29 | 15.9 | 15 | 20.3 | 14 | 13 | |
| 6 | 19 | 10.4 | 5 | 6.8 | 14 | 13 | |
| 7 | 17 | 9.3 | 5 | 6.8 | 12 | 11.1 | |
| 8 | 10 | 5.5 | 5 | 6.8 | 5 | 4.6 | |
| ≥9 | 11 | 6.0 | 4 | 5.4 | 7 | 6.5 | |
Abbreviations: BED = biological equivalent dose; ECOG PS = Eastern Cooperative Oncology Group Performance Status; RT = radiation therapy; SINS = spinal instability neoplastic score.
Other histology includes: Adrenal (n = 1), carcinoid (n = 2), colon (n = 9), dermatofibroid (n = 1), endometrium/uterine (n = 4), esophagus (n = 14), head and neck (n = 9), leiomyosarcoma (n = 1), liposarcoma (n = 1), meningioma (n = 1), mesothelioma (n = 1), neuroendocrine (n = 2), nuclear protein in testis midline carcinoma (n = 1), pancreas (n = 4), sacromatoid (n = 1), salivary (n = 1), sarcoma (n = 9), skin (n = 3), stomach (n = 2), thyroid (n = 5), and urothelial (n = 9).
Metastatic burden refers to the number of systems with metastatic disease (eg, lung, liver, etc).
Presence of neuropathic pain and paraspinal disease was unknown for 1 patient, who did not experience local failure.
Presence of epidural involvement was unknown for 25 patients, 11 of whom experienced local failure.
Multifraction regimens included 20 Gy in 5 fractions (n = 20), 30 Gy in 10 fractions (n = 95), 35 to 37.5 Gy in 14 to 15 fractions (n = 23), and other radiation therapy regimens (n = 12).
BED refers to the biological equivalent dose of the cumulative dose of RT delivered to the spine
Rates and consequences of radiographic local failure
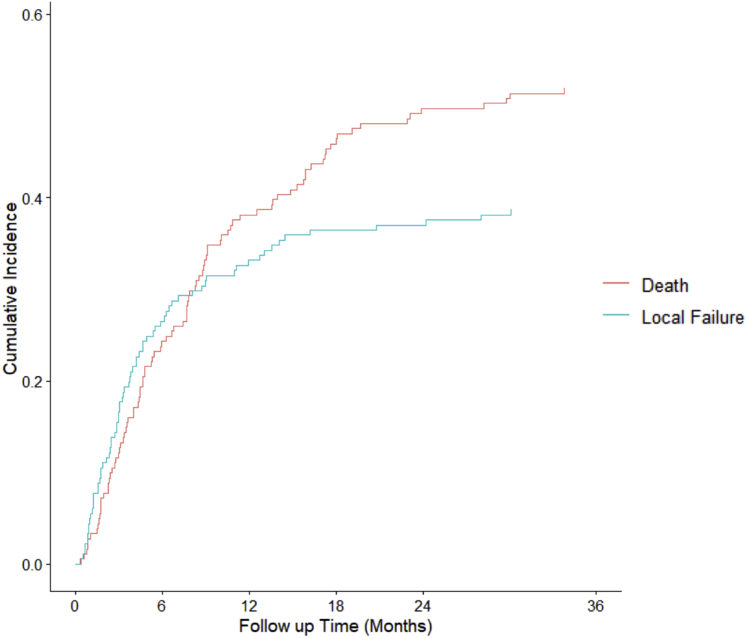
Overall, 74 of 182 patients (40.7%) experienced radiographic LF. The 6-, 12-, 18-, and 24-month LF rates were 26.5%, 33.1%, 36.5%, and 37.0% respectively, whereas corresponding rates of death were 24.3%, 38.1%, 45.9%, and 49.7% (Fig 2). Median time to LF was 3.8 months. Patients with breast histology had the lowest cumulative incidence of LF at 22.5%, followed by those with melanoma histology at 28.6%. The cumulative incidence of LF among patients with lung, kidney, prostate, and other histology was 39.5%, 80%, 53.1%, and 42%, respectively (Fig 3).
Figure 2.
Cumulative incidence of radiographic local failures versus deaths in patients with spinal metastases managed with conventional radiation therapy (n = 182).
Figure 3.
Cumulative incidence of radiographic local failures by histology in patients with spinal metastases managed with conventional radiation therapy (n = 182).
Of the 74 patients with LF, 55 (74.3%) experienced at least 1 consequence of LF. There were 38 patients (51.4%) who had new compression fractures, of which 18 (47.4%) were symptomatic. Twenty-nine patients (39.2%) had admissions for pain control, 21 patients (28.4%) developed neurologic symptoms, and 13 patients (17.6%) developed cord compression. The median time from RT to compression fracture, admission for pain control, neurologic symptoms, or cord compression was 3, 5.7, 10.5, and 9 months, respectively. Nearly half of patients with LF (41.9%) had at least 1 intervention for LF, which included reirradiation (35.1%), invasive interventions for pain control (14.9%), salvage surgery (10.8%), and vertebroplasty (6.8%). The median time from RT to reirradiation, invasive interventions for pain control, salvage surgery, and vertebroplasty was 9.2, 6, 9.6, and 3.2 months, respectively.
Predictors of local failure
On univariable analysis, histology was a significant predictor of LF (Table 2). Compared with patients with breast histology, who had the lowest cumulative incidence of LF, patients with lung (HR, 3.717; 95% confidence interval [CI], 1.638-8.435; P = .002), kidney (HR, 8.684; 95% CI, 3.286-22.948; P < .0001), and prostate histology (HR, 2.523; 95% CI, 1.115-5.708; P = .026) had significantly higher rates of LF. Those with histology other than breast, lung, kidney, prostate, and melanoma also had a higher incidence of LF (HR, 4.096; 95% CI, 1.852-9.058; P < .0001). In addition, male sex (HR, 1.731; 95% CI, 1.093-2.743; P = .019) and single-fraction RT (8 Gy) (HR, 3.172; 95% CI, 1.832-5.494; P < .0001) were significant predictors of LF. Compared with patients with SINS of 0 to 6, those with SINS of 7 to 12 and ≥13 did not have an increased risk of LF.
Table 2.
Predictors of local failure on univariable and multivariable analyses with censoring at death (n = 182)
| Variable | Univariable analyses |
Multivariable analyses |
||
|---|---|---|---|---|
| HR [95% CI] | P value | HR [95% CI] | P value | |
| Age at RT (per year) | 1.000 [0.982-1.018] | .984 | - | - |
| Male sex (Ref: female) | 1.731 [1.093-2.743] | .019 | 1.282 [0.659-2.495] | .465 |
| Time from metastatic diagnosis to RT start (per year) | 1.000 [1.000-1.000] | .485 | - | - |
| ECOG PS (Ref: 0) | ||||
| 1 | 1.156 [0.639-2.091] | .632 | - | - |
| 2 | 1.746 [0.854-3.567] | .127 | - | - |
| 3 | 1.545 [0.601-3.972] | .366 | - | - |
| Histology (Ref: breast) | ||||
| Lung | 3.717 [1.638-8.435] | .002 | 3.568 [1.532-8.309] | .003 |
| Kidney | 8.684 [3.286-22.948] | <.0001 | 4.937 [1.529-15.935] | .008 |
| Prostate | 2.523 [1.115-5.708] | .026 | 1.947 [0.680-5.572] | .214 |
| Melanoma | 1.895 [0.380-9.457] | .436 | 1.989 [0.390-10.142] | .408 |
| Other | 4.096 [1.852-9.058] | <.0001 | 3.699 [1.612-8.487] | .002 |
| Metastatic burden (Ref: 1) | ||||
| 2 | 1.323 [0.714-2.454] | .374 | - | - |
| 3 | 1.080 [0.525-2.222] | .834 | - | - |
| ≥4 | 1.860 [0.970-3.567] | .062 | - | - |
| SINS (Ref: 0-6) | ||||
| 7-12 | 0.962 [0.591-1.568] | .877 | 1.168 [0.697-1.956] | .556 |
| ≥13 | 1.069 [0.421-2.642] | .886 | 1.221 [0.483-3.083] | .673 |
| Neuropathic pain (Ref: no neuropathic pain) | 1.474 [0.920-2.361] | .106 | - | - |
| Paraspinal disease (Ref: no paraspinal disease) | 0.761 [0.458-1.263] | .290 | - | - |
| Epidural disease (Ref: no epidural disease) | 1.472 [0.856-2.532] | .162 | - | - |
| Single-fraction 8 Gy (Ref: Multifraction RT) | 3.172 [1.832-5.494] | <.0001 | 2.592 [1.437-4.675] | .002 |
Abbreviations: CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group Performance Status; HR = hazard ratio; Ref = reference group; RT = radiation therapy; SINS = spinal instability neoplastic score.
In a multivariable model (Table 2) including sex, fractionation, histology, and SINS, independent predictors of LF included single-fraction RT (8 Gy) (HR, 2.527; 95% CI, 1.397-4.571; P = .002), lung histology (HR, 3.594; 95% CI, 1.545-8.365; P = .003), kidney histology (HR, 4.888; 95% CI, 1.526-15.661; P = .008), and other histology (HR, 3.861; 95% CI, 1.676-8.894; P = .002). Age, male sex, time from metastatic diagnosis to start of RT, Eastern Cooperative Oncology Group, metastatic burden, SINS, and presence of paraspinal, neuropathic, or epidural disease were not independent predictors of LF.
Discussion
The majority of patients who develop spinal metastases undergo palliative radiation therapy to alleviate pain, prevent local disease progression, and prevent the development of subsequent pathologic fractures or neurologic complications.24,25 For long-term survivors, local control of spinal metastases is an important issue. The literature on LF among patients with spinal metastases receiving conventional EBRT is sparse. To date, the data available have focused on metastatic spinal cord compression and reirradiation as endpoints of LF, which may not sufficiently capture true radiographic LF events and their clinical sequelae.18, 19, 20, 21, 22 Other studies comparing single-fraction and multifraction RT, including a recently reported noninferiority randomized clinical trial examining ambulatory status as the primary endpoint, have focused on the value of different fractionation schemes in the context of clinical symptom relief.26 Of note, the patients in that study had poorer prognosis, as the median survival was 3 months and 37% of patients had died before the 2-month ambulatory status assessment. Our study is the first to our knowledge to comprehensively evaluate for predictors and consequences of radiographic LF within a cohort of patients with a more favorable prognosis, with a median follow-up of nearly 8 months following treatment with conventional EBRT alone for spinal metastases.
In this study using modern conventional EBRT techniques at an academic institution, the 1-year LF rate was 33.1%. Local failure for most patients occurred within the first 6 months after conventional EBRT, with a median time to LF of 3.8 months. There were notable clinical implications of LF, including new compression fractures, admissions for site-related symptoms, new neurologic symptoms, and development of spinal cord compression. Among the limited data available describing LF rates after conventional EBRT, one study of patients managed with primary surgery found a 1-year local recurrence of 69.3%.27 Only 60% of these patients received postoperative adjuvant therapy, which likely accounted for the higher incidence of LF compared with that of our cohort. More recent studies have shown improved local control rates that are comparable to those in our study. In two studies on metastatic spinal cord compression from relatively radioresistant tumors, the 1-year local control rates were 76% to 84% after 30 Gy (10 fractions) and 80% to 82% after higher doses of 37.5 Gy (15 fractions) or 40 Gy (20 fractions).28,29 In both of these studies, there were no statistically significant differences in local control with dose escalation beyond 30 Gy.
In concert with the known higher incidence of retreatment among patients receiving single fractionation,19,21,30, 31, 32, 33 we found that patients who received a single fraction of 8 Gy were more likely than those who received multiple fractions across a longer course of RT to experience LF. A meta-analysis of single-fraction versus multifraction randomized controlled trials in painful, uncomplicated bone metastases demonstrated that pain responses of single-fraction versus multifraction RT were equivalent; however, rates of retreatment were higher in the single-fraction group at 20% compared with 8% in the multifraction group.21 Of note, patients enrolled in the trials included in this meta-analysis did not have prespecified follow-up imaging, and thus may not have had radiographic detection of LF that otherwise may have been diagnosed by surveillance imaging. Although patients with poor expected survival would benefit from a shorter RT course to decrease the burden associated with daily RT visits, these data support treating with multifractionated RT for patients with a prognosis that exceeds the timeframe of risks related to LF.
In addition, our study found that histology was an independent predictor of LF. In particular, lung, kidney, or other histology (excluding breast, lung, kidney, prostate, or melanoma) were significantly associated with higher rates of LF, compared with breast histology, which is considered more radiosensitive and associated with a more favorable prognosis.34 For patients with kidney histology, the cumulative incidence of LF was highest, at 80%. Patients with lung histology and other histology had cumulative incidences of 39.5% and 42%, respectively. The findings of higher LF in patients with kidney and lung histology are likely related to radioresistance, as both renal cell carcinoma and non-small cell lung cancer are known to have increased radioresistance to conventional EBRT.35, 36, 37
These study findings have important implications for patients with spinal tumors. First, the high rates of LF occurring largely within the first 6 months post-RT highlight the critical role of prognostication in RT decision-making in the management of spinal tumors. Our data suggest that patients with a life expectancy of greater than a few months require consideration of the risks of potential LF after conventional EBRT. Furthermore, predictors of LF—namely dose and histology—should also influence treatment decisions for spinal tumors. Given that single-fraction RT is associated with an increased risk of LF among patients with favorable prognosis and that LF is related to significant clinical consequences, other RT modalities or fractionation patterns may be important to consider for patients in whom longer life expectancy is projected. Prognosis prediction models may be used to identify which patients may survive long enough to benefit from more nuanced considerations of treatments and LF outcomes.38,39 Clinicians should weigh the risk of LF and its consequences versus the risk of death in the treatment decision-making process.
For some patients with favorable prognosis, especially for those with more radioresistant tissue histology, dose-escalated radiation may be beneficial. Reports of 1-year local control estimates ranging from 81% to 98% suggest that SBRT may have a role in improving local control outcomes in spinal metastases.9,11, 12, 13,40, 41, 42, 43, 44, 45, 46, 47 A recent phase II randomized controlled trial noted improvements in pain response and local control for patients receiving SBRT of 12 or 16 Gy compared with multifraction RT of 30 Gy (10 fractions).48
Finally, these findings inform surveillance imaging after RT for spinal metastases. Given the high proportion of patients who experience LF within the first 6 months of RT, interval imaging of patients every 2 to 3 months in the first 6 months after RT may be indicated. Given continued failures after 6 months, ongoing imaging at longer intervals may also be indicated. Such imaging may facilitate early management of LF and avoid site-related events such as admissions for failure-related pain and metastatic spinal cord compression.
Limitations of this study include the retrospective nature of our review from a single institution. In addition, patients for whom we did not have at least 1 posttreatment CT or MRI available to review, from which we could determine radiologic evidence of LF, were excluded from analyses. This likely selects out the patients with very poor prognosis and patients who travel from long distances to an academic center for portions of their cancer care. Most patients without imaging had died within 4 months of RT completion, and may not have lived long enough to experience or to receive imaging to detect LF before death. Patients with very poor prognosis also may not have desired posttreatment imaging as part of their goals of care. Finally, this study was unable to distinguish between compression fracture as a consequence of LF or complication of treatment. However, the risk of treatment-related fracture is significantly less with conventional RT compared with SBRT,49 and all of the patients with compression fractures described in this study had radiographic evidence of LF. Furthermore, although a higher SINS has been shown to increase the risk for compression fracture,50 SINS was not a predictor of LF on univariable or multivariable analyses. This suggests that the compression fractures noted in this study are likely a consequence of LF and not due to treatment or intrinsic vertebral instability.
Despite the limitations, this is the first study to our knowledge to report on radiographic LF rates after conventional EBRT and is one of the largest studies to date on patients with spinal metastases treated with conventional EBRT with a long follow-up period for surviving patients. For patients who were consistently seen at our institution, we had routine interval imaging after treatment, which provided early, objective radiologic evidence of LF and thus increased accuracy of LF rates at each 6-month timepoint. These data may guide clinicians in management decisions, informing expectations, and counseling patients with spinal metastases treated with conventional EBRT.
Conclusions
Patients with spinal metastases treated with conventional EBRT experience an over 30% rate of LF by 1 year, which results in the frequent need for admissions for pain control and interventions such as reirradiation and surgery. Significant predictors of LF include single-fraction RT of 8 Gy and primary lung or kidney histology. Given the high rates of LF and its clinical consequences, our study suggests that single-fraction RT of 8 Gy should be reserved for patients with poor prognosis, and that assessment of the risk of death versus LF is important when determining treatment modality and fractionation. Further research is needed to determine how to reduce LF among high-risk patients.
Footnotes
Sources of support: This work had no specific funding.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Disclosures: none.
This work was presented as an oral presentation at the American Society for Radiation Oncology (ASTRO) 61st Annual Meeting in Chicago, IL, September 15-18, 2019.
References
- 1.Macedo F., Ladeira K., Pinho F. Bone metastases: An overview. Oncol Rev. 2017;11:321. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selvaggi G., Scagliotti G. Management of bone metastases in cancer: A review. Clin Rev Oncol Hematol. 2005;56:365–378. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Maccauro G., Spinelli M.S., Mauro S. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011 doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsamadicy A.A., Adogwa O., Lubkin D.T. Thirty-day complication and readmission rates associated with resection of metastatic spinal tumors: A single institutional experience. J Spine Surg. 2018;4:304–310. doi: 10.21037/jss.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hage W.D., Aboulafia A.J., Aboulafia D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31:515–528. doi: 10.1016/s0030-5898(05)70171-1. vii. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez R.K., Wade S.W., Reich A. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchuk I., Hutton B., Moretto P. Incidence, consequences and treatment of bone metastases in breast cancer patients-experience from a single cancer centre. J Bone Oncol. 2013;2:137–144. doi: 10.1016/j.jbo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wibmer C., Leithner A., Hofmann G. Survival analysis of 254 patients after manifestation of spinal metastases: Evaluation of seven preoperative scoring systems. Spine. 2011;36:1977–1986. doi: 10.1097/BRS.0b013e3182011f84. [DOI] [PubMed] [Google Scholar]
- 9.Bishop A.J., Tao R., Rebueno N.C. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–1026. doi: 10.1016/j.ijrobp.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Olson R.A., Tiwana M.S., Barnes M. Use of single-versus multiple-fraction palliative radiation therapy for bone metastases: Population-based analysis of 16,898 courses in a Canadian Province. Int J Radiat Oncol Biol Phys. 2014;89:1092–1099. doi: 10.1016/j.ijrobp.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen Q.N., Shiu A.S., Rhines L.D. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185–1192. doi: 10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Mehta N., Zavitsanos P.J., Moldovan K. Local failure and vertebral body fracture risk using multifraction stereotactic body radiation therapy for spine metastases. Adv Radiat Oncol. 2018;3:245–251. doi: 10.1016/j.adro.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patchell R.A., Tibbs P.A., Regine W.F. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 14.Klimo P., Thompson C.J., Kestle J.R.W. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunning E.C., Butler J.S., Morris S. Complications in the management of metastatic spinal disease. World J Orthop. 2012;3:114–121. doi: 10.5312/wjo.v3.i8.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao A., Sarkiss C.A., Ladner T.R. Contemporary spinal oncology treatment paradigms and outcomes for metastatic tumors to the spine: A systematic review of breast, prostate, renal, and lung metastases. J Clin Neurosci. 2017;41:11–23. doi: 10.1016/j.jocn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Ciftdemir M., Kaya M., Selcuk E. Tumors of the spine. World J Orthop. 2016;7:109–116. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow E., van der Linden Y.M., Roos D. Single versus multiple fractions of repeat radiation for painful bone metastases: A randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15:164–171. doi: 10.1016/S1470-2045(13)70556-4. [DOI] [PubMed] [Google Scholar]
- 19.Harstell W.F., Scott C.B., Bruner D.W. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 20.Steenland E., Leer J., Van Houwelingen H. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;97:798–804. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 21.Chow E., Harris K., Fan G. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 22.Rades D., Stalpers L.J.A., Veninga T. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23:3366–3375. doi: 10.1200/JCO.2005.04.754. [DOI] [PubMed] [Google Scholar]
- 23.Fisher C.G., Dipaola C.P., Ryken T.C. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the spine oncology study group. Spine. 2010;35:E1221–E1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 24.Gerszten P.C., Mendel E., Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine. 2009;34(22 Suppl):S78–S92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]
- 25.Laufer I., Bilsky M.H. Advances in the treatment of metastatic spine tumors: The future is not what it used to be. J Neurosurg Spine. 2019;30:299–307. doi: 10.3171/2018.11.SPINE18709. [DOI] [PubMed] [Google Scholar]
- 26.Hoskin P.J., Hopkins K., Misra V. Effect of single-fraction vs multifraction radiotherapy on ambulatory status among patients with spinal canal compression from metastatic cancer: The SCORAD randomized clinical trial. JAMA. 2019;322:2084–2094. doi: 10.1001/jama.2019.17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klekamp J., Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998;140:957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 28.Rades D., Freundt K., Meyners T. Dose escalation for metastatic spinal cord compression in patients with relatively radioresistant tumors. Int J Radiat Oncol Biol Phys. 2011;80:1492–1497. doi: 10.1016/j.ijrobp.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Freundt K., Meyners T., Bajrovic A. Radiotherapy for oligometastatic disease in patients with spinal cord compression (MSCC) from relatively radioresistant tumors. Strahlentherapie und Onkol. 2010;186:218–223. doi: 10.1007/s00066-010-2110-9. [DOI] [PubMed] [Google Scholar]
- 30.Lutz S., Balboni T., Jones J. Palliative radiation therapy for bone metastases: Update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Wu J.S.Y., Wong R., Johnston M. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 32.Lovelock D.M., Zhang Z., Jackson A. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77:1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarnold J.R. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- 34.Yamada Y., Katsoulakis E., Laufer I. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6. doi: 10.3171/2016.9.FOCUS16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Meerleer G., Khoo V., Escudier B. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:e170–e177. doi: 10.1016/S1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 36.Lesueur P., Lequesne J., Barraux V. Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer) Radiat Oncol. 2018;13:138. doi: 10.1186/s13014-018-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh P.K., Faivre-Finn C., Blackhall F.H. Targeted agents in non-small cell lung cancer (NSCLC): Clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev. 2012;38:626–640. doi: 10.1016/j.ctrv.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Chow E., Abdolell M., Panzarella T. Predictive model for survival in patients with advanced cancer. J Clin Oncol. 2008;26:5863–5869. doi: 10.1200/JCO.2008.17.1363. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan M.S., Epstein-Peterson Z., Chen Yu-Hui. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer. 2014;120:134–141. doi: 10.1002/cncr.28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar R., Nater A., Hashmi A. The era of stereotactic body radiotherapy for spinal metastases and the multidisciplinary management of complex cases. Neuro-Oncol Pract. 2015;3:48–58. doi: 10.1093/nop/npv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishop A.J., Tao R., Guadagnolo B.A. Spine stereotactic radiosurgery for metastatic sarcoma: Patterns of failure and radiation treatment volume considerations. J Neurosurg Spine. 2017;27:303–311. doi: 10.3171/2017.1.SPINE161045. [DOI] [PubMed] [Google Scholar]
- 42.Miller J.A., Balagamwala E.H., Berriochoa C.A. The impact of decompression with instrumentation on local failure following spine stereotactic radiosurgery. J Neurosurg Spine. 2017;27:436–443. doi: 10.3171/2017.3.SPINE161015. [DOI] [PubMed] [Google Scholar]
- 43.Chang E.L., Shiu A.S., Mendel E. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y., Bilsky M.H., Lovelock D.M. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Leeman J.E., Bilsky M., Laufer I. Stereotactic body radiotherapy for metastatic spinal sarcoma: A detailed patterns-of-failure study. J Neurosurg Spine. 2016;25:52–58. doi: 10.3171/2015.11.SPINE151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerszten P.C., Burton S.A., Ozhasoglu C. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 47.Laufer I., Iorgulescu J.B., Chapman T. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J Neurosurg Spine. 2013;18:207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen Q.N., Chun S.G., Chow E. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: A randomized phase 2 trial. JAMA Oncol. 2019;5:872–878. doi: 10.1001/jamaoncol.2019.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahgal A., Whyne C.M., Ma L. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14:e310–e320. doi: 10.1016/S1470-2045(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 50.Fisher C.G., Schouten R., Versteeg A.L. Reliability of the spinal instability neoplastic score (SINS) among radiation oncologists: An assessment of instability secondary to spinal metastases. Radiat Oncol. 2014;9:69. doi: 10.1186/1748-717X-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]