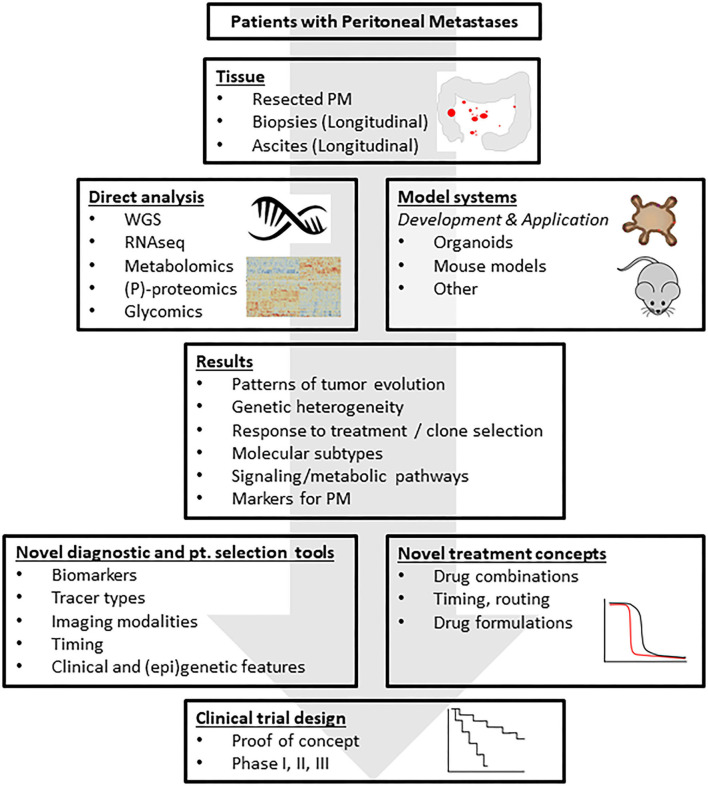
Figure 2.
A research framework addressing the challenges. The standardized collection of tissue and body fluids derived from patients with peritoneal metastases from CRC generates biobanks of frozen and fixed tissues for downstream molecular analysis. Moreover, organoid technology allows the co-establishment of “living biobanks” in which individual cancer patients are represented by their tumor-derived organoids. These organoids may be used in transplantation studies generating spontaneous metastasis models in mice. The molecular tissue analyses will generate novel leads for the detection and treatment of PM, for understanding resistance mechanisms, tumor cell plasticity and intra- and inter-lesion (epi-)genetic heterogeneity. Insight into the biology of PM will lead to the formulation of novel treatment concepts that can subsequently be tested in the generated novel PM model systems. These efforts should yield a series of pre-clinically validated novel treatment strategies, possibly limited to specific, identifiable patient subgroups. These strategies can then be tested in small proof-of-concept studies to generate biological proof for the validity of the treatment concept in cancer patients, and subsequently in regular phase 1–3 clinical trials. Analysis of the tissues from such trials may subsequently identify potential resistance mechanisms and help design pre-clinical studies that are aimed at further improving the strategy. This translational feed-forward approach has the potential to impact clinical outcome in the years to come. PM, peritoneal metastases; WGS, whole genome sequencing; RNAseq, RNA sequencing; (P)-proteomics, (phospho-)proteomics.