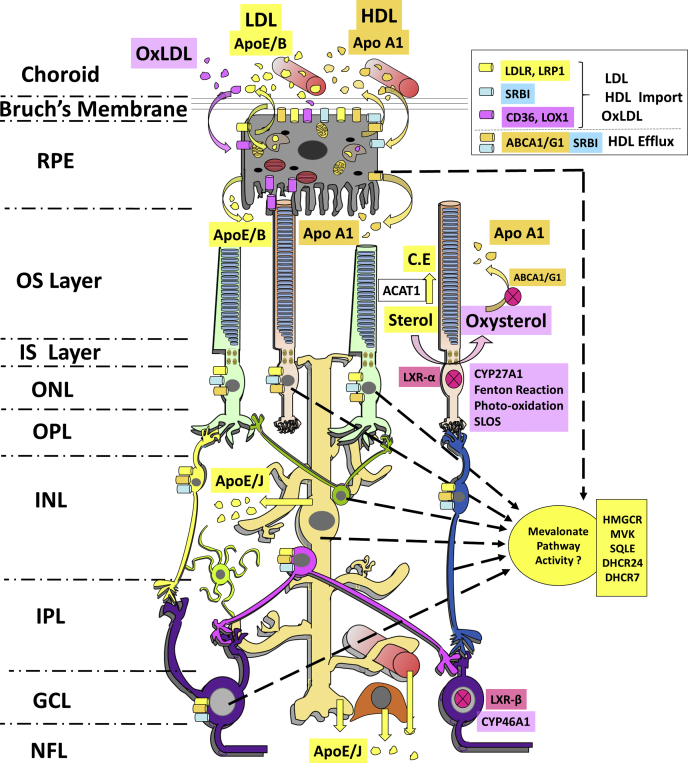
Fig. 3.
Hypothetical model of cholesterol homeostatic processes governing the vertebrate retina. The mevalonate pathway is active in both the RPE and the neural retina; however, the exact contributions of each of the retinal cell types to the overall synthesis and steady-state content of cholesterol in the retina remains to be determined. The RPE is capable of ABCA1-mediated bidirectional sterol efflux. The RPE also may exhibit apical secretion of APO-E–containing LDL, as well as LDLR-dependent uptake of LDL, and CD36-dependent uptake of OxLDL from the choroid. CD36 is also involved in diurnal uptake of rod outer segment (OS) tips; however, lipid hydroperoxides and oxysterols may competitively inhibit this process. Müller glia actively synthesize, package (with APO-E and APO-J), and secrete cholesterol, which then can be taken up by neighboring neurons. Sterol efflux from the neural retina is dependent on the activities of CYP27A1, CYP46A1, LXRs, and ABCA1. Excess retinal cholesterol may be esterified and stored as lipid droplets by the activity of ACAT1 and LCAT. Oxidative stress, involving both enzymatic and nonenzymatic processes, can lead to oxysterol formation; those by-products either are removed from the cell by sterol efflux or remain and accumulate in lipid droplets and cellular membranes, which can result in retinal pathology. (See Fig. 2 and text for definition of abbreviations.)