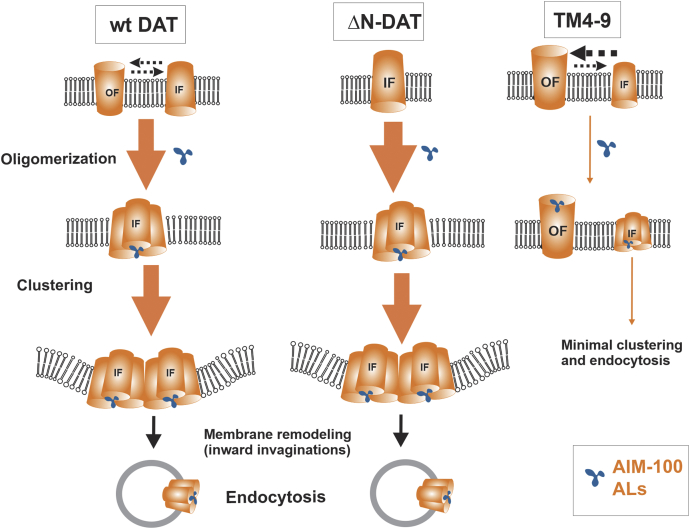
Figure 10.
Hypothetical model of the effects of AIM-100 and ALs on DAT. AIM-100 and ALs stabilize trimers (oligomers), promote formation of high-order oligomers, and nanoclusters of a fraction of WT DAT in the IF conformation and ΔN-DAT, that is predominantly in the IF state. Clustering of concave-shaped DATs leads to membrane remodeling and stabilizing inward invaginations of the membrane, followed up by vesicle scission (endocytosis). The same ALs and AIM-100 are less efficient in inducing oligomerization of DAT in the OF conformation. For example, the capacity of AIM-100 and ALs to promote oligomerization of TM4-9 is diminished because of the increased probability of the OF state of this mutant. AIM-100 and ALs, are, however, still capable of binding at or near the substrate binding sites of the TM4-9 mutant in its OF state. Widths of arrows roughly reflect process probabilities. AL, AIM-100–like; DAT, dopamine transporter; IF, inward-facing; OF, outward-facing; TM, transmembrane.