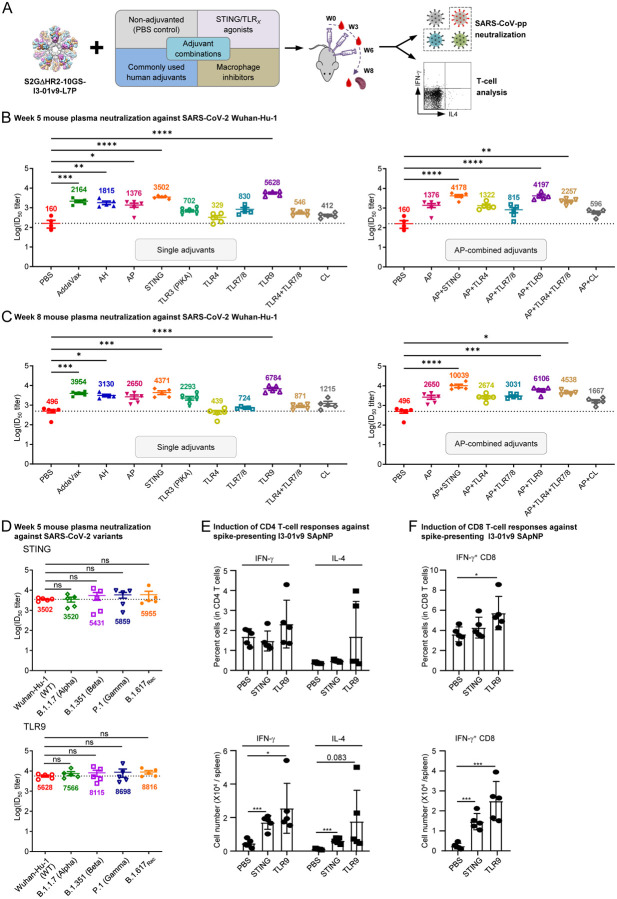
Fig. 2. Adjuvants enhance the I3–01v9 SApNP vaccine -induced plasma neuralization of both the wildtype strain and four variants.
(A) Schematic representation of mouse immunization with the I3–01v9 SApNP with diverse adjuvant formulations and functional assessment by SARS-CoV-2-pp neutralization assays and T-cell analysis. Conventional adjuvants, STING/TLR agonists, macrophage inhibitors, and adjuvant combinations were compared to non-adjuvanted control (PBS). (B, C) Mouse plasma neutralization against the wildtype SARS-CoV-2 strain, Wuhan-Hu-1, at weeks 5 and 8 after two and three footpad injections, respectively. ID50 titers derived from SARS-CoV-2-pp neutralization assays are plotted, with average ID50 values labeled on the plots. (D) Neutralization against four variants by mouse plasma from STING (top) and CpG (bottom)-formulated vaccine groups. ID50 titers derived from SARS-CoV-2-pp neutralization assays are plotted. Neutralization data were analyzed using either one-way ANOVA (B-C) or repeated measures one-way ANOVA (D) to compare ID50 titers. Dunnett’s multiple comparison post hoc test was performed. Splenic mononuclear cells derived from mice in the STING and CpG groups (n = 5 mice/group) at week 8 were cultured in the presence of BALB/C DCs pulsed with I3–01v9 SApNP (1 × 10−7 mM). Cells were harvested 16 h following re-activation. (E) Production of IFN-γ-producing Th1 CD4+ T cells and IL-4-producing Th2 CD4+ T cells. (F) IFN-γ-producing CD8+ effector T cells. T-cell responses were analyzed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. ns (not significant), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.