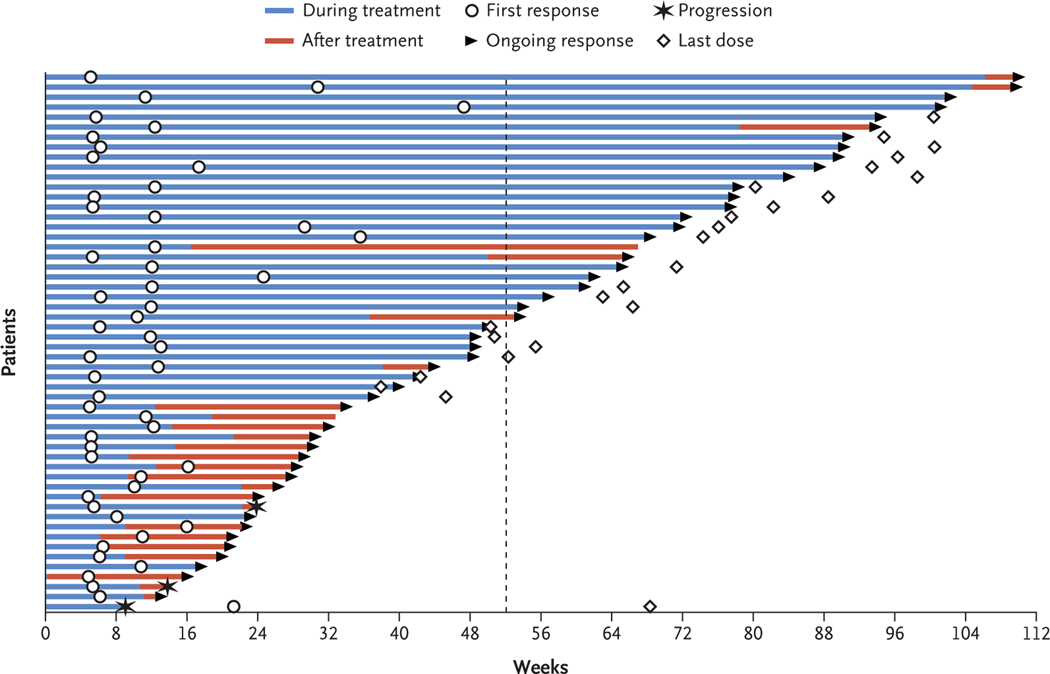
Figure 1. Time to and Duration of Intracranial Response.
The plot shows the onset and durability of intracranial objective responses to the combination of nivolumab and ipilimumab, according to modified Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, criteria. Open circles indicate the first evidence of objective response (complete or partial response), and arrows indicate an ongoing response; 47 of 52 responses (90%) were ongoing at the time of the analysis. The dashed line indicates 1 year after treatment initiation. The median time to response was 2.3 months (range, 1.1 to 10.8). The median duration of intracranial response has not been reached.