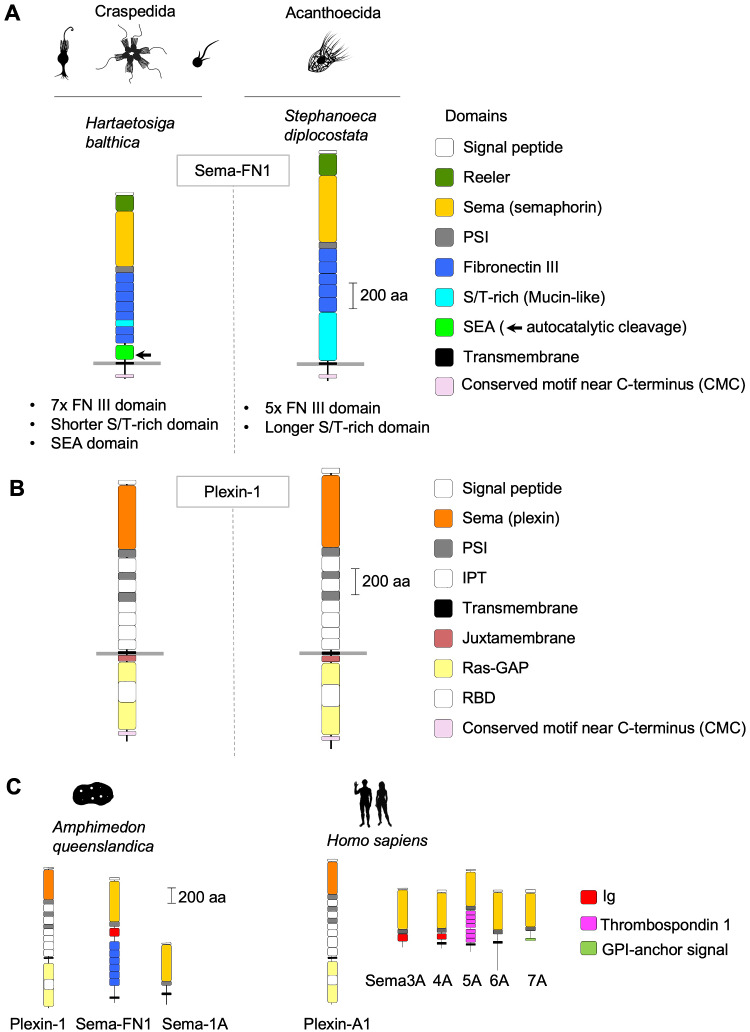
Fig. 1.
Domain architecture of choanoflagellate semaphorins and plexins. (A) Domain architecture of Sema-FN1, shown for one Craspedida and one Acanthoecida species. The N-terminal Reeler domain is unique for choanoflagellate semaphorins and not found in metazoan semaphorins. It is followed by a Sema and a PSI domain. The extracellular stalk contains 7 FNIII domains in Craspedida and 5 in Acanthoecida. A SEA domain with autocatalytic cleavage site (arrow) is only present in Craspedida Sema-FN1. The serine/threonine (S/T)-rich domain of variable size is predicted to be the site of O-linked glycosylation. A CMC motif containing ∼10 conserved amino acid positions is found close to the C-terminus. (B) Domain architecture of Plexin-1 is conserved in choanoflagellates and nearly identical to Metazoa plexins, except for the presence of a CMC motif with similarity to the CMC of Sema-FN1. Note that the Signal peptides (N-terminal), and IPT (extracellular) and RBD domains (intracellular) are colored in white. (C) Representative examples of plexin and semaphorin orthologs of Metazoa, shown for a sponge species (Amphimedon queenslandica) and for man (Homo sapiens). Silhouette pictures of organisms are from phylopic.org.