Figure 4. PAL-1 localizes to the mitochondria and labels complex I.
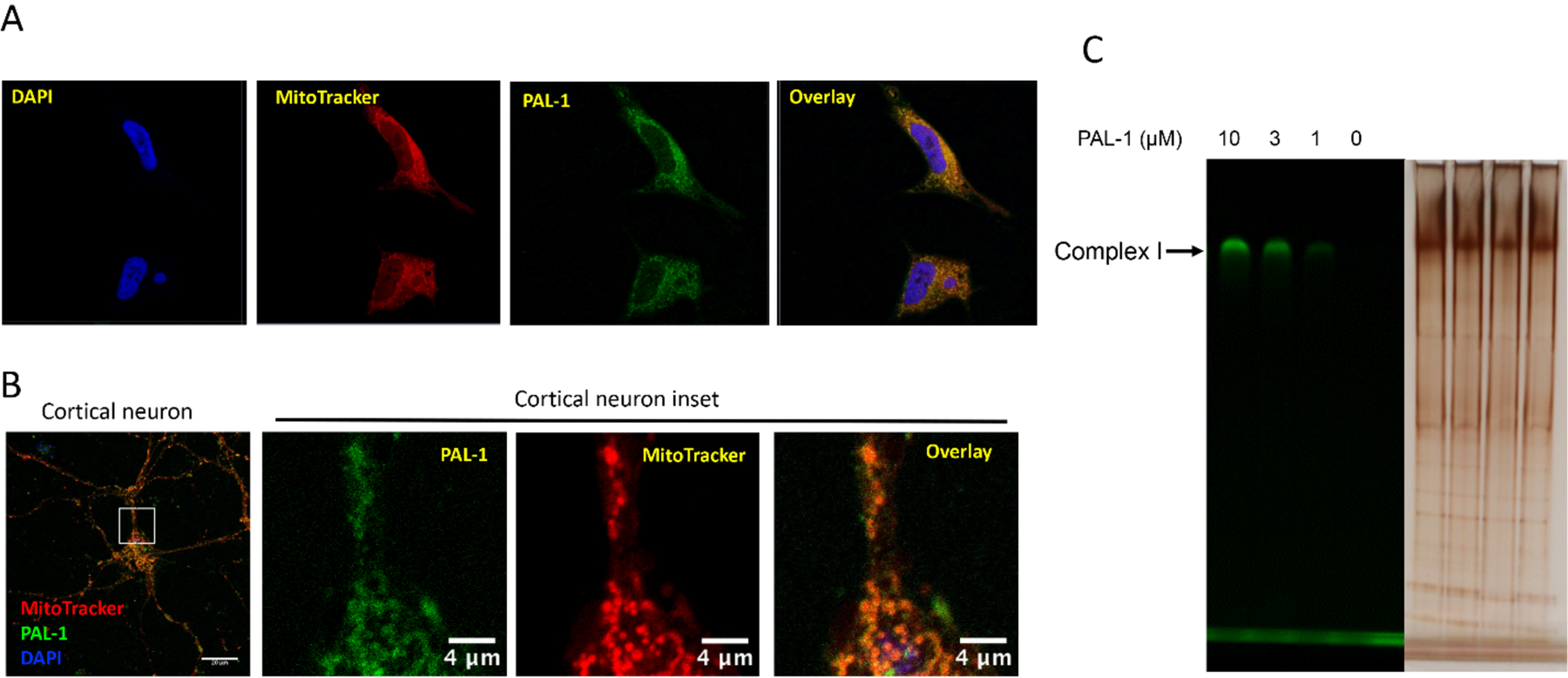
A) MC65 cells were treated with PAL-1 (3 μM) for 3.5 h. After incubation with MitoTracker Red (300 nM) and DAPI (2 μg/mL), samples were fixed and imaged with confocal microscopy. Image adjustments are as follows: DAPI unmodified, MitoTracker Red gamma adjusted to 1.15, PAL-1 NBD channel white level reduced to 13000, black increased to 350; B) Cultured neurons (DIV 10 to 12) were incubated with PAL-1 (25 μM) for 4 h, then incubated with Mitotracker Red CMXRos (25 nM) for 30 min. Neurons were washed × 3 by ½ media exchanges with neuronal culture media without phenol red. Neurons were imaged live on a Zeiss LSM710 confocal laser scanning microscope using a 63x/1.4 NA oil-immersion objective with pin hole of 1 Airy disc unit and Nyquist sampling; C) Mouse brain mitochondria (0.1 mg) were incubated with DMSO or PAL-1 at indicated concentrations for 1 h at 4 °C. The samples were UV irradiated at 365 nm for 10 min. After lysis, the samples were resolved by colorless Native PAGE and the gel was imaged with a BioRad imager system with excitation of 488 nm;
