Figure 2.
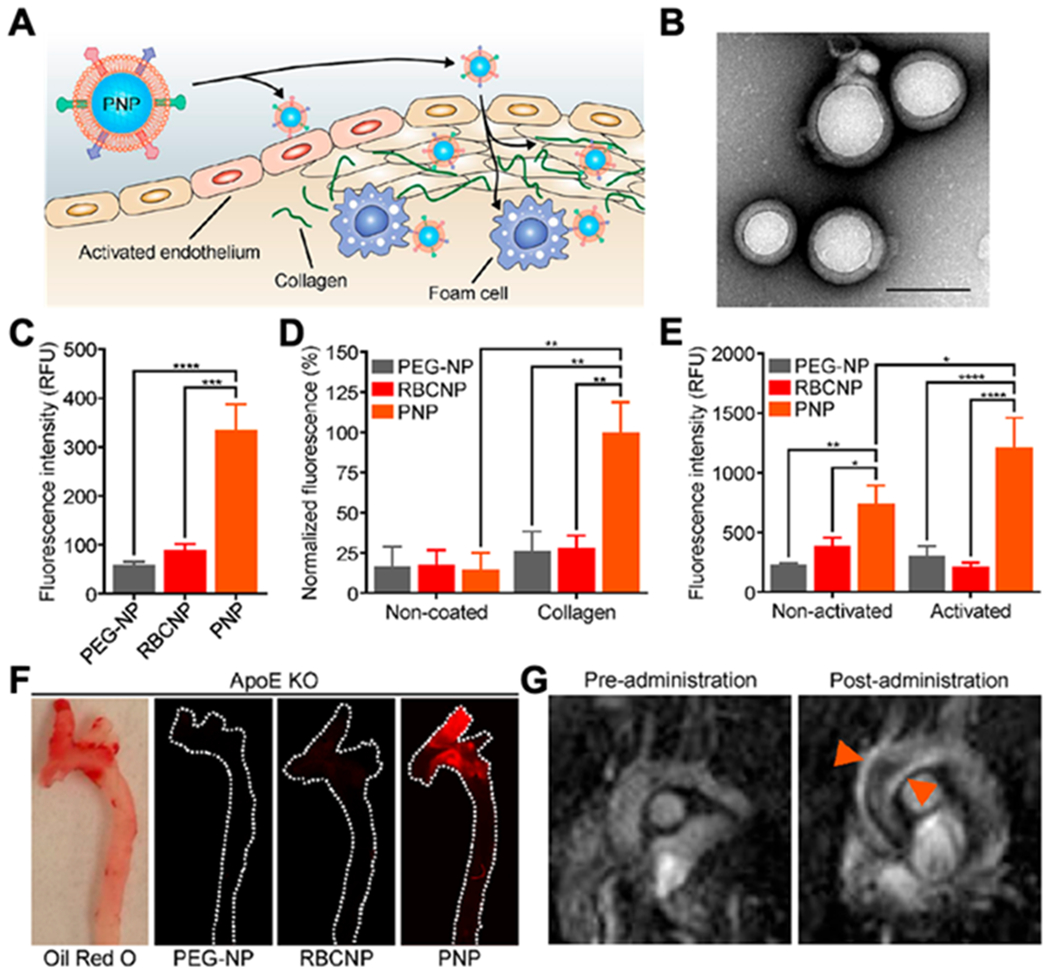
Platelet membrane-coated nanoparticles (PNPs) for targeting and detection of atherosclerosis. (A) Schematic illustration of PNPs targeting different components of the atherosclerotic plaque, including activated endothelium, collagen and foam cells. (B) Transmission electron microscopy (TEM) image of PNPs negatively stained with uranyl acetate (scale bar of 100 nm). (C) Quantification of PLGA nanoparticles functionalized with polyethylene glycol (PEG-NPs), PLGA nanoparticles coated with red blood cell membrane (RBCNPs) or PNPs bound to foam cells measured by flow cytometry (n = 3, mean ± SD). All formulations were made with PLGA cores labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD) dye. (D) Fluorescent quantification of PEG-NPs, RBCNPs, or PNPs bound to non-coated or collagen-coated surfaces (n = 3, mean ± the standard error of the mean). (E) Fluorescent quantification of PEG-NPs, RBCNPs, or PNPs bound to human umbilical vein endothelial cells (HUVEC) in the resting state or after activation with tumor necrosis factor alpha (TNF-α) (n= 3, mean ± SD). (F) Fluorescent imaging of aortic arches from ApoE knockout (ApoE KO) mice fed on a high-fat western diet after intravenous administration with PEG-NPs, RBCNPs, or PNPs (white line represents the physical outline of the aortic arch and red fluorescence represents nanoparticles). Oil Red O staining was used to confirm the presence of plaque in the mice. (G) T1-weighted magnetic resonance imaging (MRI) ofApoE KO mice before and 1 hour after administration with PNPs loaded with gadolinium, an MRI contrast agent (orange arrows point to regions of positive contrast in the aortic arch). The statistical analysis was performed using one-way analysis of variance (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Reproduced with permission. [27] Copyright 2018, The American Chemical Society.
