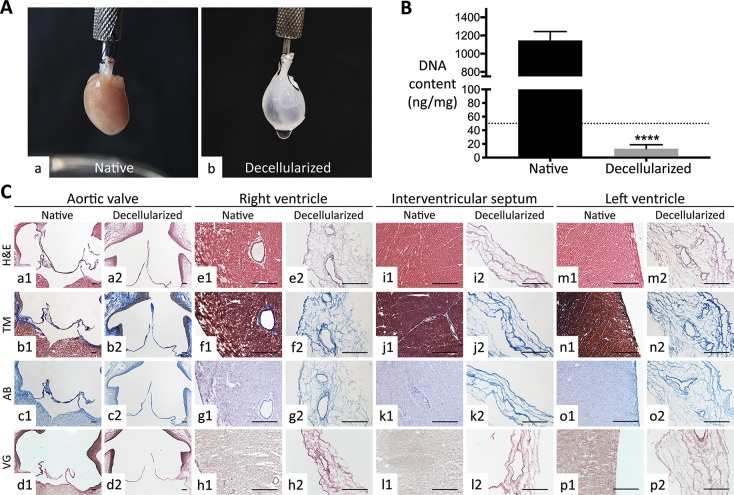
Figure 1.
Decellularization yield of whole rat hearts. (A) Macroscopic view of (a) native and (b) decellularized hearts. Note the discoloration and the natural ECM scaffold (auricles, interventricular septum, coronary arterial tree) rendered visible at naked eye after decellularization. (B) DNA quantification pre- and postdecellularization. Decellularized hearts possessed a significantly lower DNA content with respect to native ones. Data are expressed as media ± standard deviation (****, p < 0.0001). The value of DNA residue for treated hearts was under the threshold of 50 ng/mg (dotted line) defined for decellularization by Crapo et al.37 (C) Histological evaluation of organ architecture pre- and post-decellularization. Valve apparatus (e.g., aortic valve), right and left ventricles, and interventricular septum of decellularized hearts showed a conserved ECM at Haematoxylin-Eosin (H&E; a2, e2, i2, and m2), Masson trichrome (MT; b2, f2, j2, and n2), Alcian Blue (AB; c2, g2, k2, and o2), and elastic Van Gieson (VG; d2, h2, l2, and p2) with respect to their native counterpart (a1–d1, e1–h1, i1–l1, and m1–p1). The original morphology of aortic valve cusps was well preserved after decellularization. Because of the high cellularity of both ventricles and interventricular septum, decellularization induced an important reduction of tissue thickness. Magnification bars: 200 μm for the aortic valve (a1–d1 and a2–d2)); 100 μm for the other tissues (e1–p1 and e2–p2).