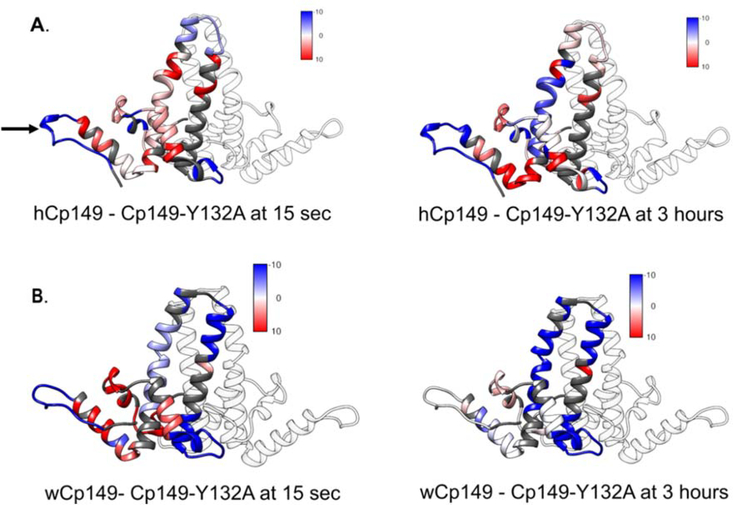
Figure 5.
Mutation at residue 132 alters exchange across Cp149. A. The difference in the amount of deuterium taken up by the wild type and Cp149-Y132A human dimers at 15 seconds (left) and 3 hours (right). Areas where the wild type dimer takes up more deuterium than the Cp149-Y132A mutant dimer are displayed in red. Areas where the mutant Cp149-Y132A dimer takes up more deuterium than the wild type dimer are displayed in blue. Regions with no difference are displayed in white and regions that were not reported in this experiment are displayed in gray. B. The difference in the amount of deuterium taken up by the wild type and Cp149-Y132A woodchuck dimers at 15 seconds (left) and 3 hours (right). The location of the Y132A mutation is shown with an arrow in panel A.