Abstract
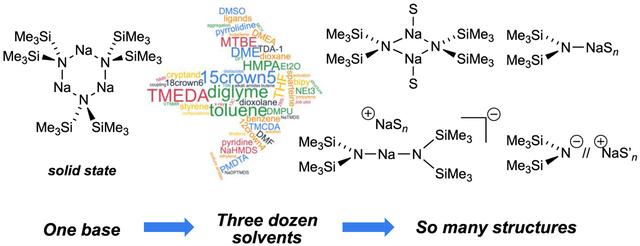
We report solution structures of sodium hexamethyldisilazide (NaHMDS) solvated by >30 standard solvents (ligands). These include: toluene, benzene, and styrene; triethylamine and related trialkylamines; pyrrolidine as a representative dialkylamine; dialkylethers including THF, tert-butylmethyl ether, and diethyl ether; dipolar ligands such as DMF, HMPA, DMSO, and DMPU; a bifunctional dipolar ligand nonamethylimidodiphosphoramide (NIPA); polyamines N,N,N ,N -tetramethylenediamine (TMEDA), N,N,N ,N″,N″-pentamethyldiethylenetriamine (PMDTA), N,N,N ,N -tetramethylcyclohexanediamine (TMCDA), and 2,2 -bipyridine; polyethers 12-crown-4, 15-crown-5, 18-crown-6, and diglyme; 4,7,13,16,21,24-hexaoxa-l,10-diazabicyclo[8.8.8]hexacosane ([2.2.2] cryptand); and tris[2-(2-methoxyethoxy)ethyl]amine (TDA-l). Combinations of 1H, 13C, 15N, and 29Si NMR spectroscopies, the method of continuous variations, X-ray crystallography, and density functional theory (DFT) computations reveal ligand-modulated aggregation to give mixtures of dimers, monomers, triple ions, and ion pairs. 15N 29Si coupling constants distinguish dimers and monomers. Solvation numbers are determined by a combination of solvent titrations, observed free and bound solvent in the slow exchange limit, and DFT computations. The relative abilities of solvents to compete in binary mixtures often match that predicted by conventional wisdom but with some exceptions and evidence of both competitive and cooperative (mixed) solvation. Crystal structures of a NaHMDS cryptate ion pair and a 15-crown-5-solvated monomer are included. Results are compared with those for lithium hexamethyldisilazide, lithium diisopropylamide, and sodium diisopropylamide.
Graphical Abstract
INTRODUCTION
As part of ongoing efforts to pique the community’s interest in organosodium chemistry1 by probing structure reactivity selectivity relationships, we have put considerable effort into removing multiple stigmas associated with sodium diisopropylamide (NaDA).2,3 By contrast, there are no such constraints placed on sodium hexamethyldisilazide (NaHMDS). It is arguably the pre-eminent organosodium reagent in both academic and industrial laboratories,4 finding applications requiring nuanced control of regio-, stereo-, and chemo-selectivity.5 Its solubility, stability, and commercial availability appeal to synthetic chemists and render NaHMDS an attractive target for studying aggregation and solvation. Aside from NaHMDS crystal structures of potential interest to synthetic chemists (Chart 16), there are remarkably few physicochemical studies of NaHMDS in solution.6d, 7,10 Even the computational community has shown little interest.11
Chart 1.
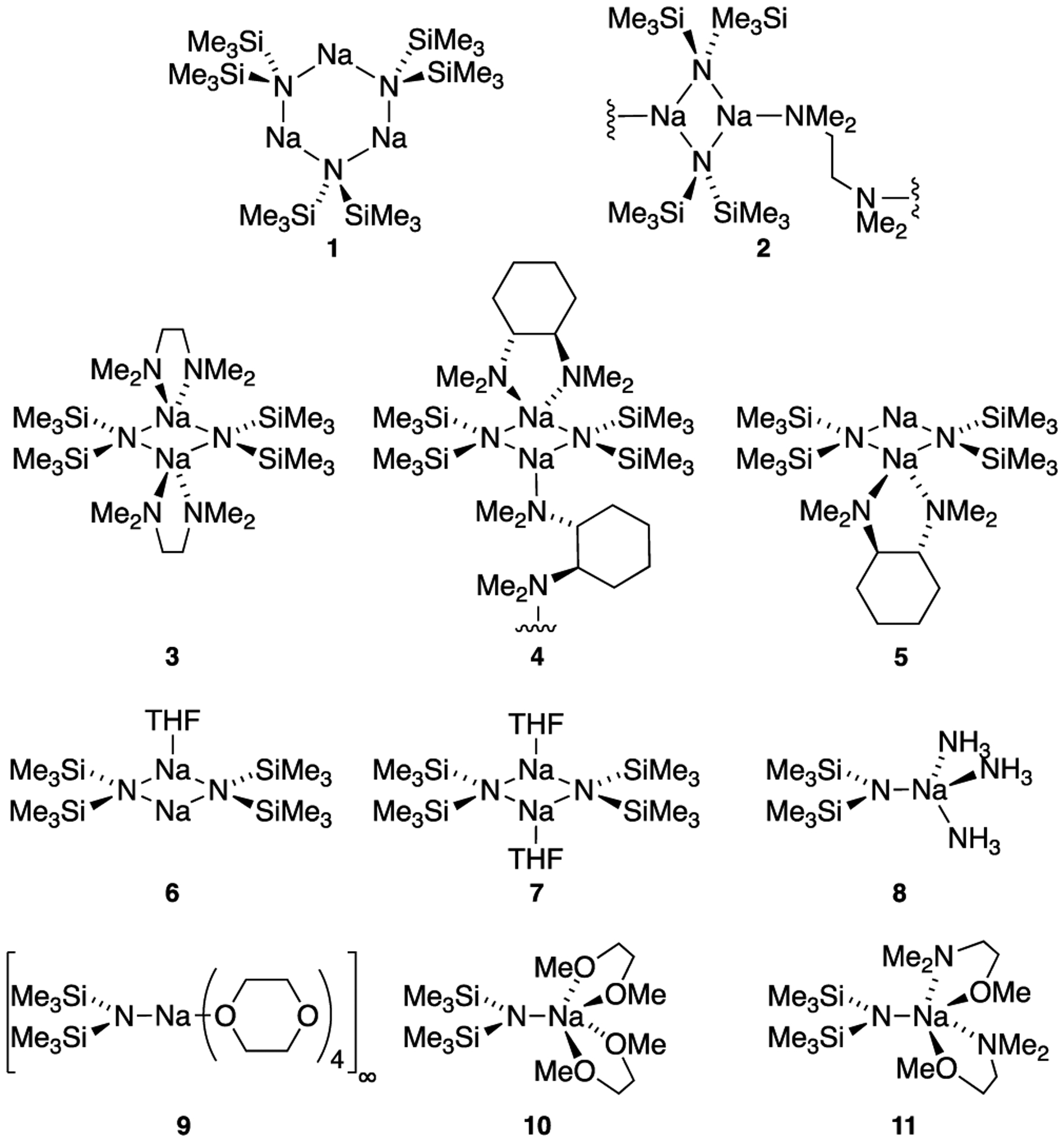
X-ray Structures of Homoleptic NaHMDS Aggregates Solvated by Synthetically Standard Solvents6
We describe herein NMR spectroscopic and computational studies of NaHMDS coordinated by several dozen mono-, bi-, and polyfunctional solvents. We use the terms “ligand” and “solvent” interchangeably. Our intention is to establish structural foundations for subsequent studies of solvent-dependent reactivities and selectivities. A secondary but still important goal is to provide a compendium of NaHMDS solvent combinations to prompt potential consumers to think beyond the standard solvents. Highly solvent-dependent structures offer a potential opportunity for practitioners to optimize selectivities and reactivities by targeting the underlying structures.12
RESULTS AND DISCUSSION
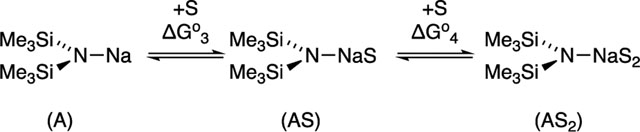
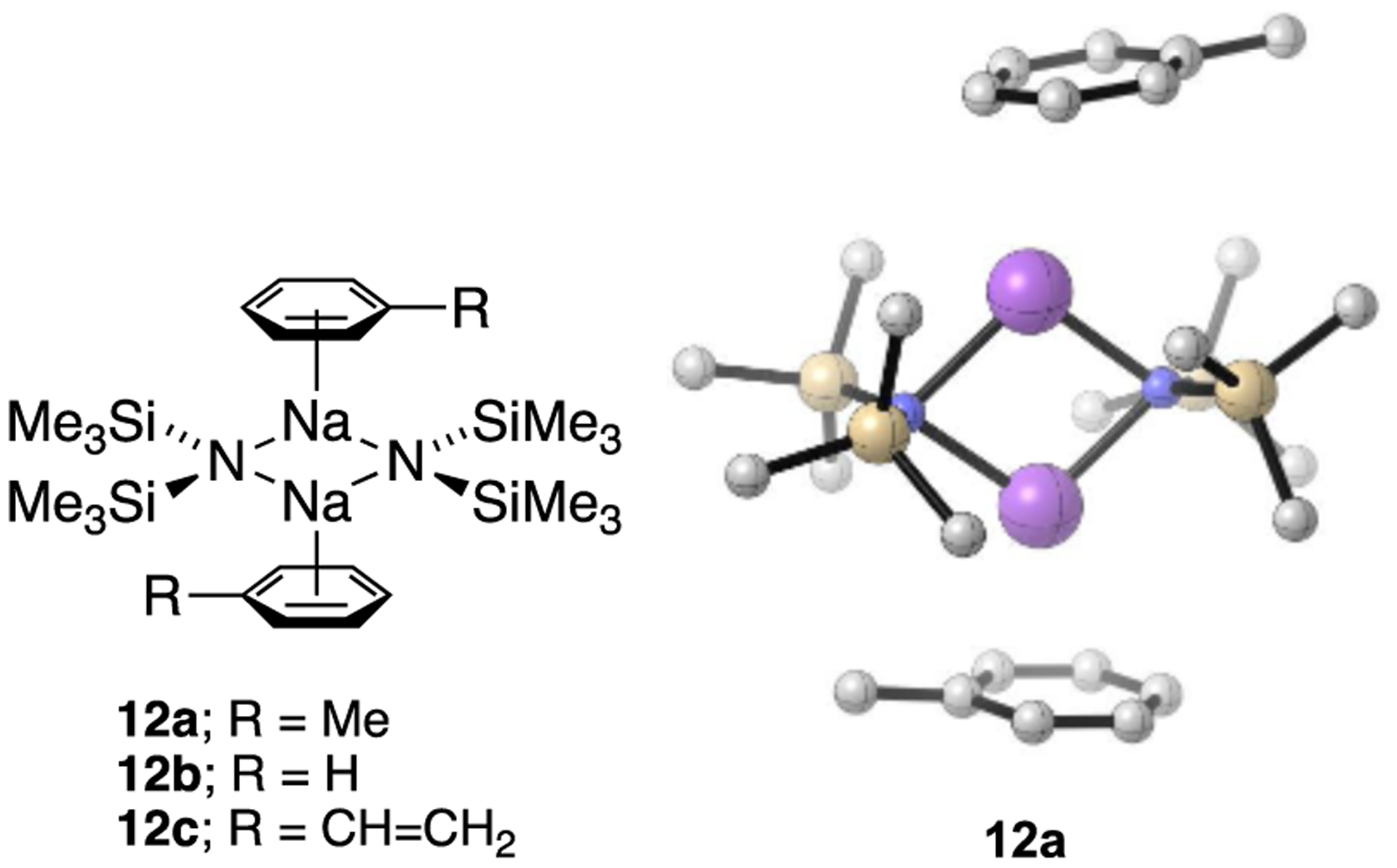
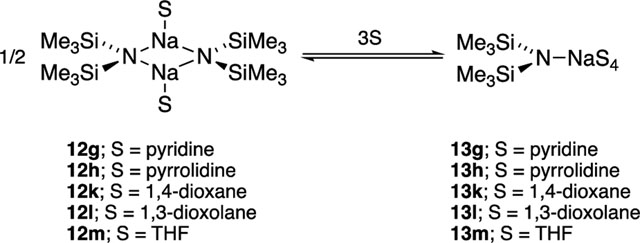
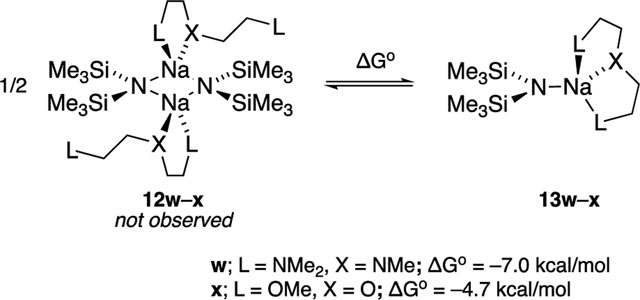
Results from spectroscopic and computational studies are summarized in Table 1 and Chart 2. The lettered entries in Table 1 also designate the coordinated solvent on numbered structures throughout. Dimer 12 and monomer 13 are dominant. Occasionally, dipolar and polydentate solvents at elevated concentrations cause phase separations, the appearance of upfield 29Si resonances as broad mounds, or the complete disappearance of 29Si signals. A chromatographically characterized cryptate ion pair allows us to attribute aberrant spectroscopic behavior to simple ion pairs (14). Triple ion 16 is observed in several instances. We observed mixed solvates 15 on many occasions owing to the titration protocols routinely employed, as documented in the Supporting Information; only those observed to the exclusion of homosolvated dimers are included in Table 1. The Supporting Information also includes significant data and pertinent undiscussed observations.
Table 1.
Spectroscopic and Computational Data for NaHMDS Dimers and Monomers 12 16, Chart 2) Different Solventsa
| entry | solvent | structure (AmSn ( )) |
29Si shifts (ppm (1JN Si)) |
solvation energy per S Na (kcal/mol) |
|---|---|---|---|---|
| a | toluene | A2S2 (12a) | 14.4 (7.9) | 1.6 |
| b | benzene | A2S2 (12b) | 14.2 (7.8) | 2.3 |
| c | styrene | A2S2 (12c) | 14.3 (7.8) | 1.5 |
| d | DMEA | A2S2 (12d) | 15.7 (8.7) | 5.9 |
| e | Et3N | A2S2 (12e) | 14.5 (7.6) | 4.1 |
| f | N-Me-pyrrolidine | A2S2 (12f) | 15.5 (8.7) | 6.7 |
| g | pyridine | A2S2 (12g) | 15.4 (8.8) | 7.3 |
| AS3 (13g) | 20.7 (13.0) | 5.5 | ||
| h | pyrrolidine | A2S2 (12h) | 15.5 (9.2) | 8.6 |
| AS4 (13h) | 19.7 (11.1) | 7.4 | ||
| i | Et2O | A2S2 (12i) | 15.5 (8.3) | 5.0 |
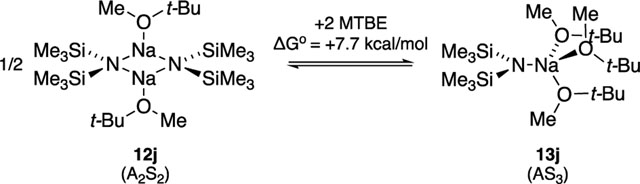
| j | MTBE | A2S2 (12j) | 15.6 (8.8) | 7.7 |
| k | 1,4-dioxane | A2S2 (12k) | 6.5 | |
| AS3 (13k) | 6.0 | |||
| l | 1,3-dioxoIane | A2S2 (12l) | 16.0 (8.6) | |
| AS4 (13l) | 21.1 (13.0) | |||
| m | THF | A2S2 (12m) | 15.8 (8.7) | 6.7 |
| AS4 (13m) | 21.7 (13.3) | 5.5 | ||
| n | HMPA | A2S2 (12n) | 16.5 (9.8) | 13.6 |
| AS3 (13n) | 23.7 (13.4) | 11.46 | ||
| o | DMPU | A2S2 (12o) | 16.4 | 10.9 |
| AS2 (13o) | 22.5 (13.6) | 13.1 | ||
| p | DMF | A2S4 (12p) | 8.2 | |
| AS4 (13p) | 22.4 (13.7) | 9.3 | ||
| q | DMSO | A2S4 (12q) | 6.5 | |
| AS3 (13q) | 20.0 (12.4) | 10.1 | ||
| r | TMEDA | A2S2 (12r) | 14.2 (5.8) | 7.4 |
| AS2 (13r) | 21.0 (11.3) | 9.5 | ||
| s | (R,R)-TMCDA | A2SS (15s) | 15.7 (7.7) | 11.0 |
| 14.7 (7.1) | ||||
| t | ( )-sparteine (25) | |||
| u | bipy | A2S (26u) | 16.2 (8.8) | |
| 20.5 (12.5) | ||||
| A2S2 (12u) | 15.5 (7.8) | 13.2 | ||
| v | DME | A2S2 (12v) | 14.8 (7.6) | 9.6 |
| AS2 (13v) | 21.4 (13.7) | 11.7 | ||
| w | PMDTA | AS (13w) | 22.7 (13.7) | 25.7 |
| x | diglyme | AS (13x) | 21.3 (12.7) | 23.4 |
| y | 12-crown-4 | AS (13y) | 22.4 (13.7) | 18.7 |
| z | 15-crown-5 | AS (13z) | 22.1 (13.0) | 29.4 |
| aa | 18-crown-6 | A2S (16aa) | 22.3 (13.6) | |
| AS (13aa) | 21.6 (12.3) | 28.8 | ||
| bb | TDA-1 (31) | A2S (16bb) | 20.5 (13.5) | |
| AS (13bb) | 19.7 (12.8) | 23.8 | ||
| cc | [2.2.2]crypt (32) | A2S (16cc) | 20.5 (13.5) | |
| AS (14cc) | 27.2 |
Details of the cosolvent and temperatures are found in the Supporting Information.
Chart 2.
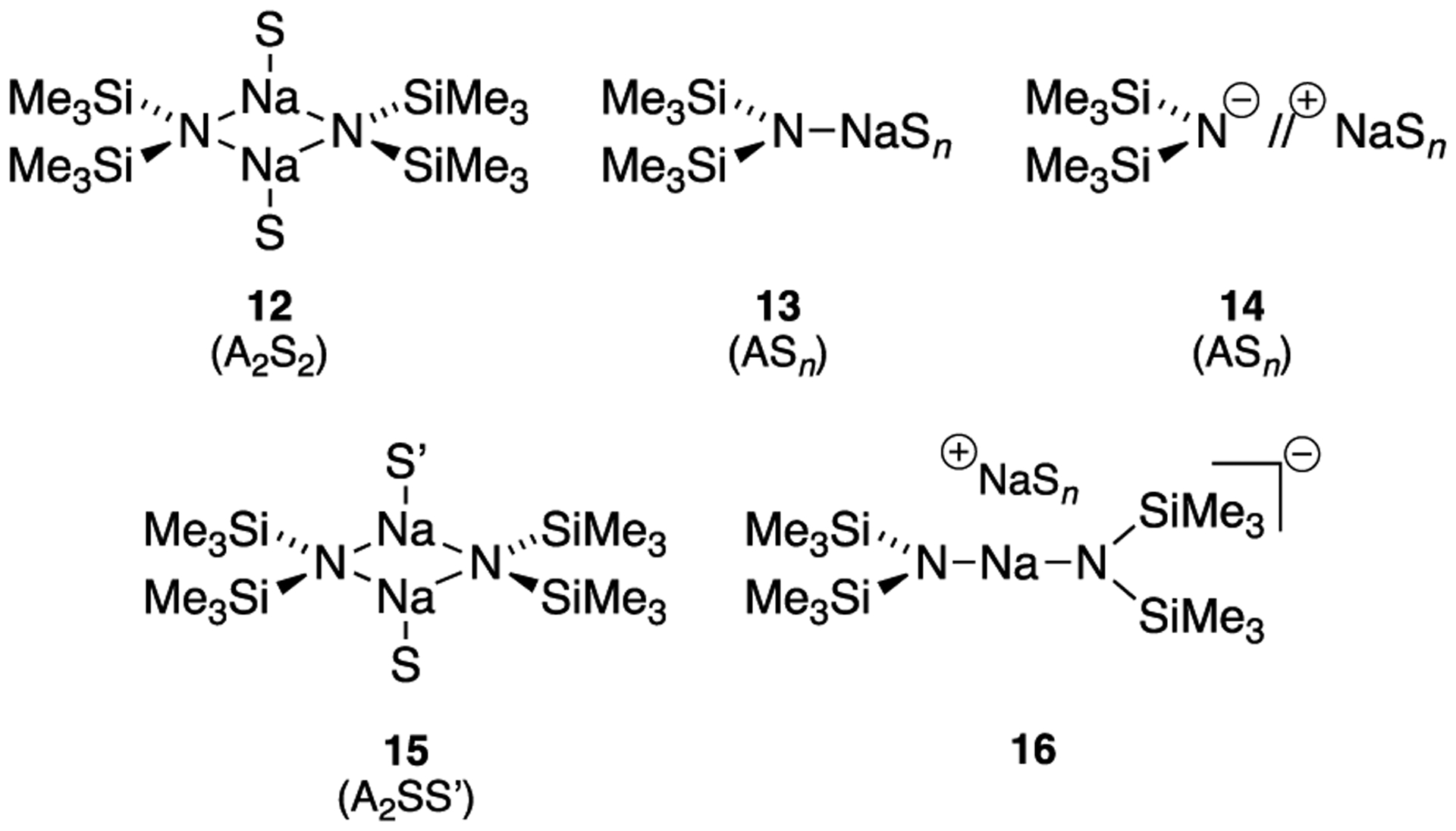
Structural Forms of NaHMDS
This paper begins with general discussions of tactics and protocols for studying aggregation and solvation using a few results emblematically. Data are presented only to illustrate the protocols, making no attempt to adjudicate every assignment. General protocols are followed by results from the various mono-, di-, tri-, and polyfunctional solvents (ligands). These assignments are compared to those of lithium hexamethyldisilazide (LiHMDS),13,15 lithium diisopropylamide (LDA),13,16 and sodium diisopropylamide (NaDA).3 We conclude by considering binary mixtures of solvents with studies of relative binding affinities that inadvertently revealed cooperative solvation effects. Computed structures of sodium cations may prove useful in understanding ionizations.
General Methods.
NaHMDS, [l5N]NaHMDS, sodium tetramethyldisilazide (NaTMDS, 17)17 sodium bis(dimethyl-(phenyl)silyl)amide (NaDPTMDS, 18),18 and sodium disilazide 1919 were prepared as white crystalline solids from the disilazanes and sodium metal. The synthesis of (Me3Si)215NH has also been reduced from 8 days20 to 4 h with improved yields. Toluene is used routinely as a cosolvent, but it is not an innocent spectator as discussed below. Substitutionally labile N N-dimethylethylamine (DMEA), 2:1 pentane/toluene-d8, and tert-butylmethyl ether (MTBE) were used as cosolvents to record spectra at 120 °C or 110 °C and to optimize solubilities and spectral resolution. No attempt is made to justify or clarify the choice of cosolvent on a case-by-case basis, deferring details to the Supporting Information. 1H, 13C, 15N, and 29Si NMR spectroscopies offered complementary perspectives; 13C and 29Si NMR spectroscopy proved most important.
Density functional theory (DFT) computations probe spectroscopically derived structural assignments and experimentally elusive details of solvation. They were carried out at the M06-2X level of theory.20,22 The standard Def2-SVP basis set was used for geometric optimizations and the expanded Def2-TZVP basis set for single point calculations.23,24 Prompted by a recent publication revealing consequential free energy changes with larger integration grid sizes,25 geometric optimizations and single-point calculations employ a refined (99,590) grid. Based on a number of comparisons, the expansion of the grid size has some influence.26 The molecular solvation events commonplace in alkali amide chemistry require computational procedures that invoke explicit solvation models instead of using an implicit solvation model. Employing both explicit and implicit solvation models can lead to large statistical errors as shown by Houk.27
Method of Continuous Variations.
The preferences for NaHMDS to form dimers at low solvent loadings and monomers at high solvent loadings (12 and 13, Chart 2) are shown using a combination of strategies. The method of continuous variations (MCVs) relies on pairing structurally similar species to afford ensembles of homo- and heteroaggregates that are characteristic of the homoaggregates28 as illustrated for dimers in eq 1. Heteroaggregates displaying 2:1 and 1:2 stoichiometries characteristic of trimers29 are not observed under any conditions. Monomers observed at elevated ligand concentrations do not heteroassociate.
Pairing partners in MCV are chosen to maximize the NMR spectroscopic resolution while maintaining structural similarity. 13C and 29Si NMR spectroscopies were used to monitor the ensemble in eq 1. 1H NMR spectroscopy was viable but the least effective. 15N NMR spectroscopy using [l5N]NaHMDS was supportive but would require both partners be labeled for optimization and was not needed.
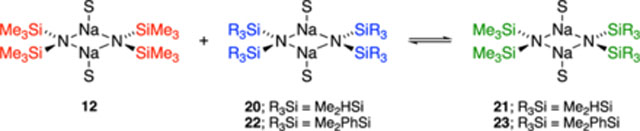
NaHMDS and its pairing partners 18 and 19 exist as homodimers in weakly coordinating solvents or at low concentrations of strongly coordinating mono- and difunctional solvents. Results from toluene (Table 1, entry a) illustrate the characterization of dimer ensembles. NaHMDS is insoluble in saturated hydrocarbons but readily dissolves as a 0.10 M solution in 2:1 pentane/toluene at 120 °C. Mixtures of [15N]NaHMDS and NaTMDS contain homodimers 12 and 20 and heterodimer 21 (Figure 1). Heterodimer 21 displays 13C resonances corresponding to the methyl resonances of the TMS and DMS groups in 3:2 proportions, reflecting the number of methyl groups (Figure 1a), a single 15N resonance
 |
(1) |
(Figure 1b); and a pair of 29Si resonances in a 1:1 proportion (Figure 1c). 15N 29Si coupling distinguishes 29Si resonances of the [15N]HMDS and unlabeled TMDS fragments and, more importantly, provides critical structural insights (discussed below).
Figure 1.
NMR spectra of 1:1 mixtures (0.30 M total titer) of [15N]NaHMDS and NaTMDS (18) in 2:1 MTBE/toluene recorded at 80 °C show homo- and heterodimers 12, 20, and 21 (eq 1): (a) 13C{1H} NMR (toluene-d3, 125.79 MHz) spectrum; (b) 15N NMR (toluene-d3, 60.66 MHz) spectrum; and (c) 29Si NMR (toluene-d3, 99.36 MHz) spectrum.
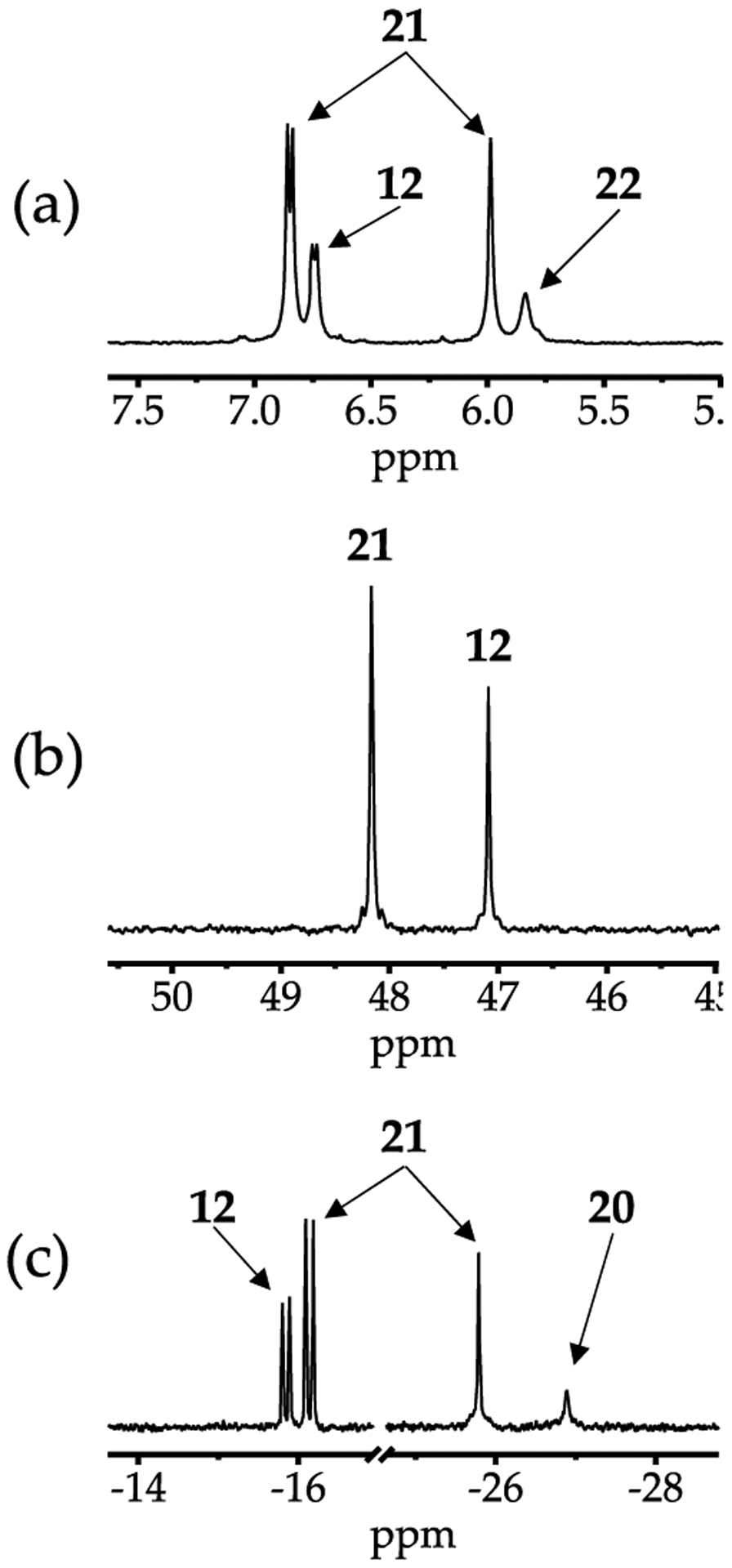
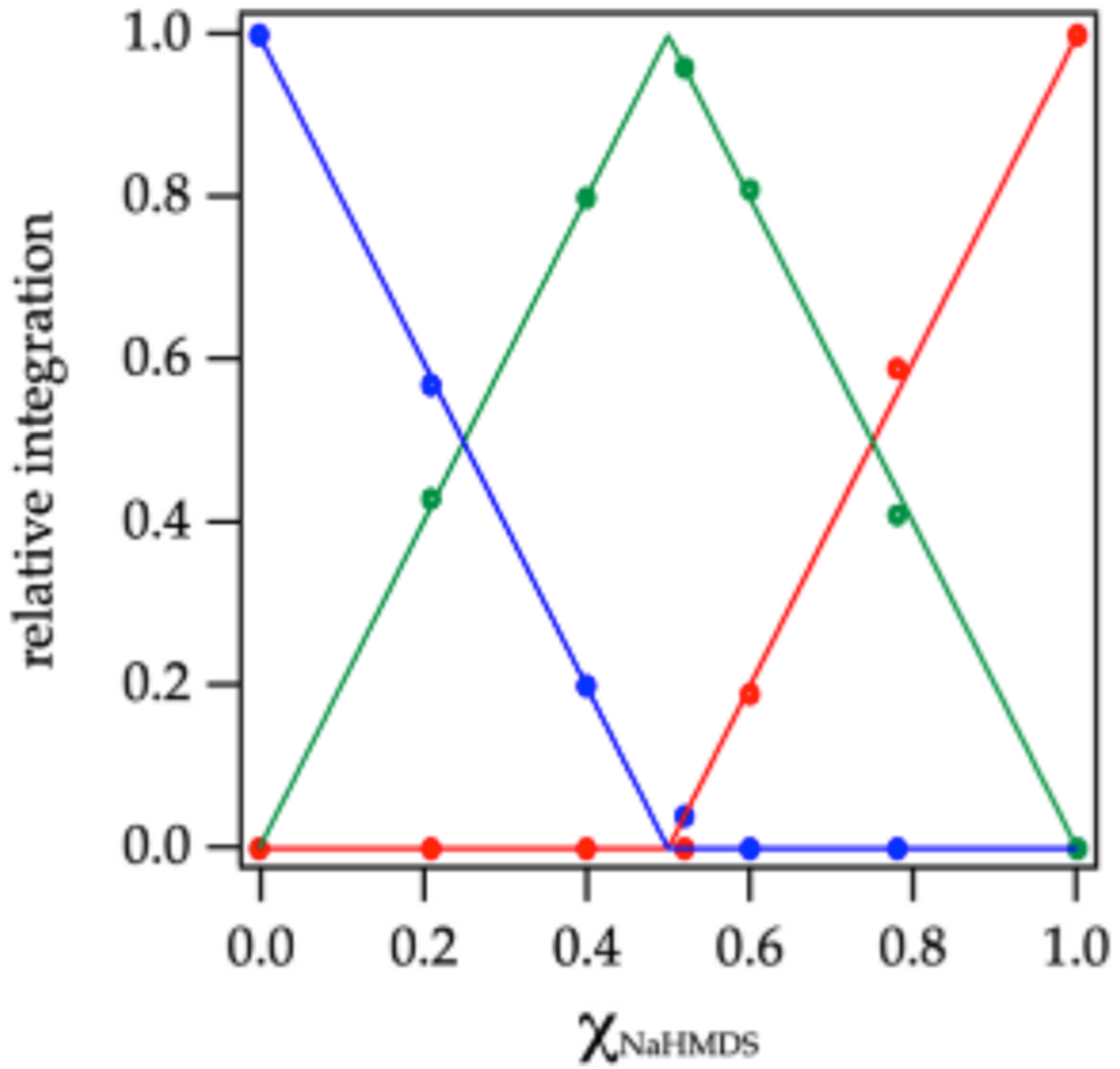
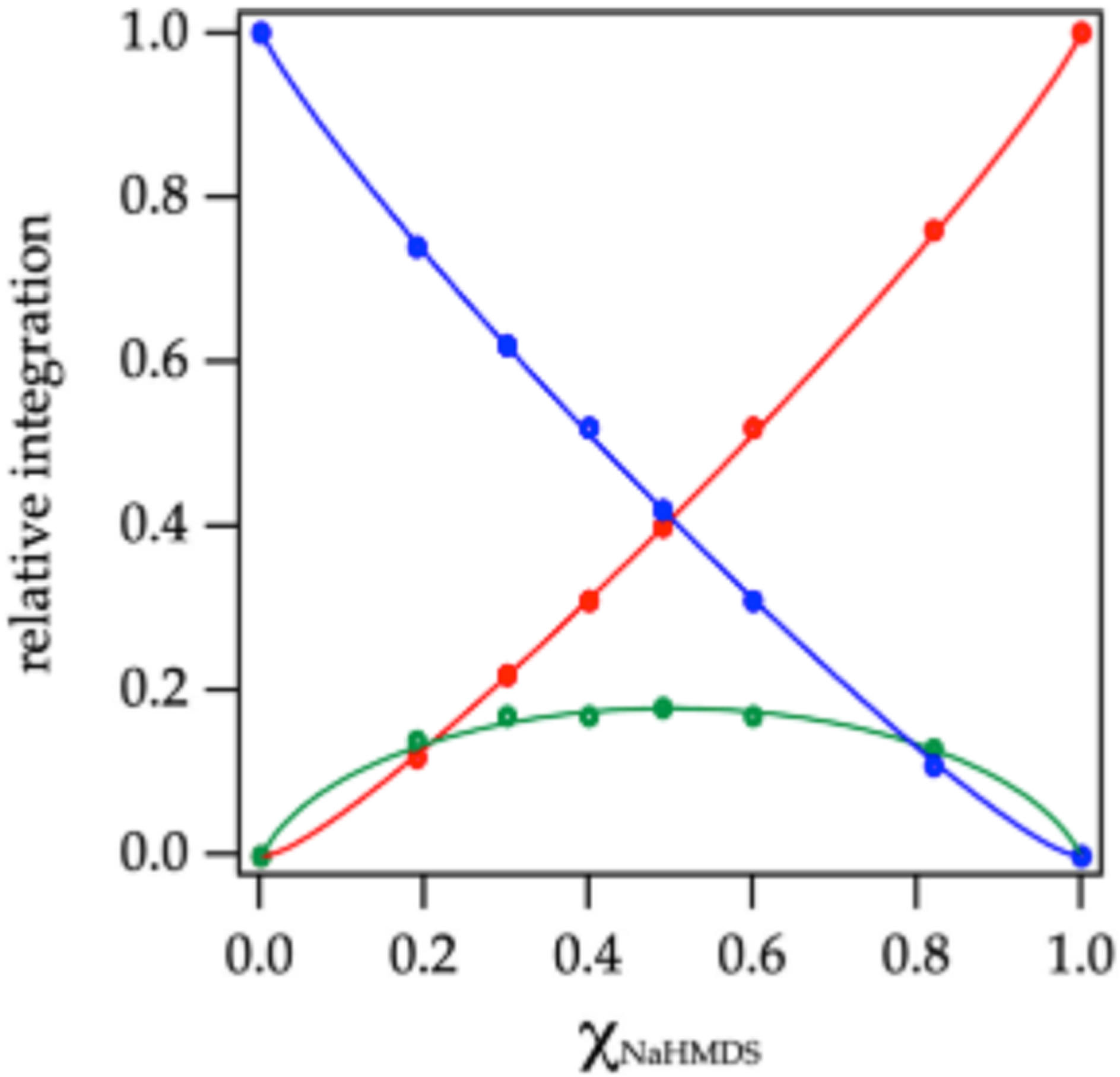
Plotting the relative concentrations of the homo- and heterodimers versus measured mole fraction31 of NaHMDS (XNaHMDs) a ords a Job plot30 showing near quantitative heterodimerization (Figure 2). The nonstatistical preference for heterodimer observed in a number of solvents is supported computationally and presumably derives from relief of congestion in the NaHMDS homodimer 12. On the other hand, using MTBE/toluene a ords a statistical Job plot as shown in Figure 3. The lower preference for heterodimerization can be attributed to a greater solvation energy of the NaHMDS and NaTMDS homodimers, which is supported computationally.
Figure 2.
Job plot showing relative integrations of the 13C{1H} resonances of NaHMDS homodimer 12 (red), NaTMDS-derived homodimer 20 (blue), and heterodimer 21 (green; eq 1) versus the measured29 mole fraction of NaHMDS (XNaHMDS) at 0.30 total molarity31 in neat toluene at 80 °C. Reprinted from Woltornist, R. A.; Collum, D. B. Using 15N 29Si Scalar Coupling to Determine Aggregation and Solvation States. J. Am. Chem. Soc. 2020, 142, 6852. Copyright 2020 American Chemical Society.
Figure 3.
Job plot showing relative integrations of the 13C{1H} resonances of NaHMDS dimer 12 (red), disilazide dimer 20 (blue), and heterodimer 21 (green; eq 1) versus the measured29 mole fraction of NaHMDS (XNaHMDS) at 0.30 M total molarity in 2:1 MTBE/toluene at 80 °C.
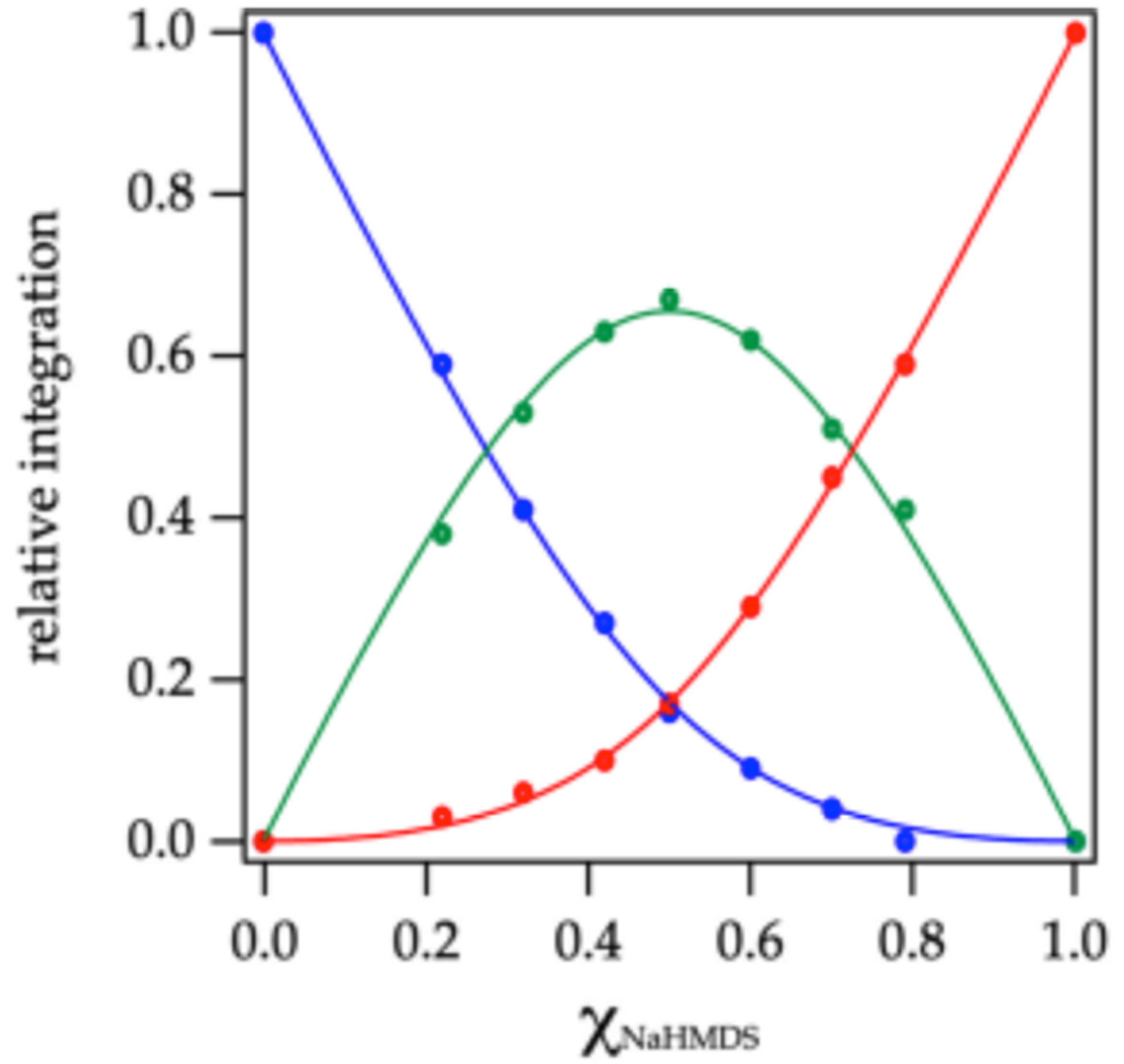
Pairing NaHMDS with the phenyl-containing sodium disilazide 18 unexpectedly favors homodimerization as illustrated in the Job plot in Figure 4. DFT computations of the corresponding homodimer drawn generically as 22 reveal a marked canting of the four phenyl moieties toward the sodium nuclei. We attribute this preference for homo dimerization to a stabilizing cation interaction unique to mixing partner 18, although this is not confirmed computationally.3
Figure 4.
Job plot showing relative integrations of the 13C{1H} resonances of NaHMDS homodimer 12 (red), NaDPTMDS-derived homodimer 22 (blue), and heterodimer 23 (green; eq 1) versus the measured29 mole fraction of NaHMDS (XNaHMDs) at 0.30 total molarity31 in neat toluene at 80 °C.
Solvent-Dependent Deaggregation.
Elevated concentrations of all but the most poorly coordinating solvents cause NaHMDS to deaggregate. Dimer monomer exchanges are rapid at 80 °C and slow at 120 °C. The clearest view of solvent-concentration-dependent structural changes is derived from HMPA as illustrated in Scheme 1 and Figure 5. The NaHMDS monomers show no associated forms when NaHMDS is mixed with disilazides 18 and 19, whereas the triple ions 16 form heteroaggregated triple ions.
Scheme 1.
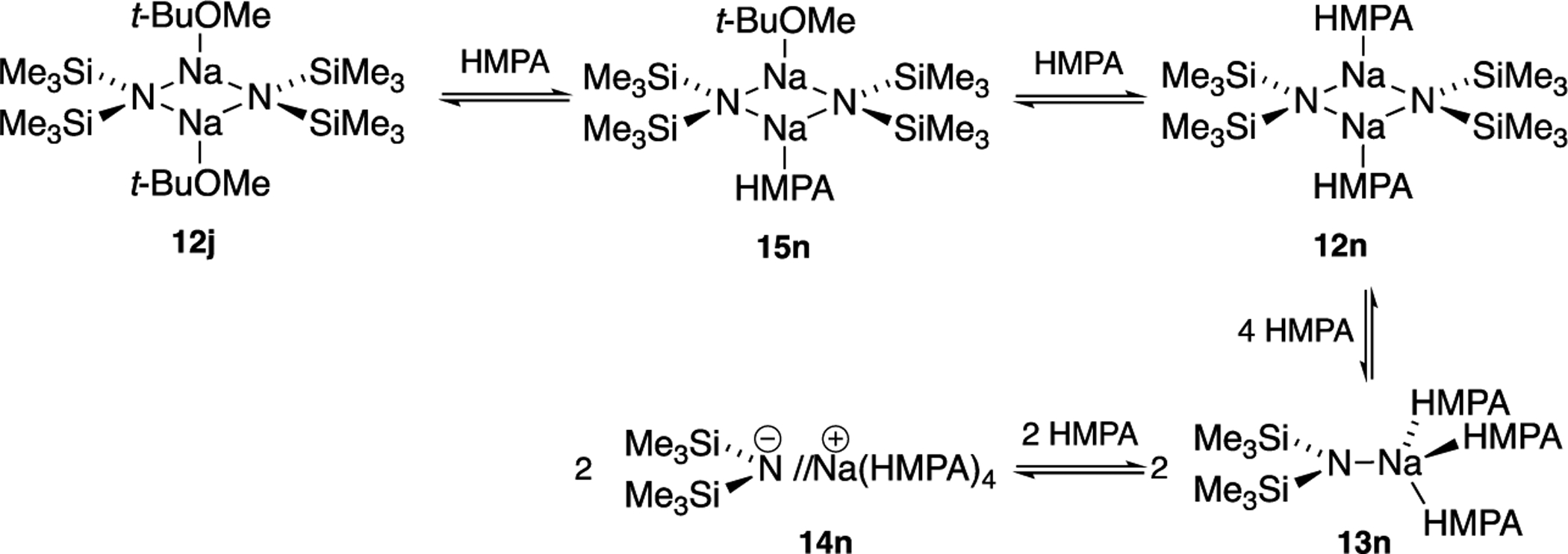
Serial Solvation by HMPA
Figure 5.
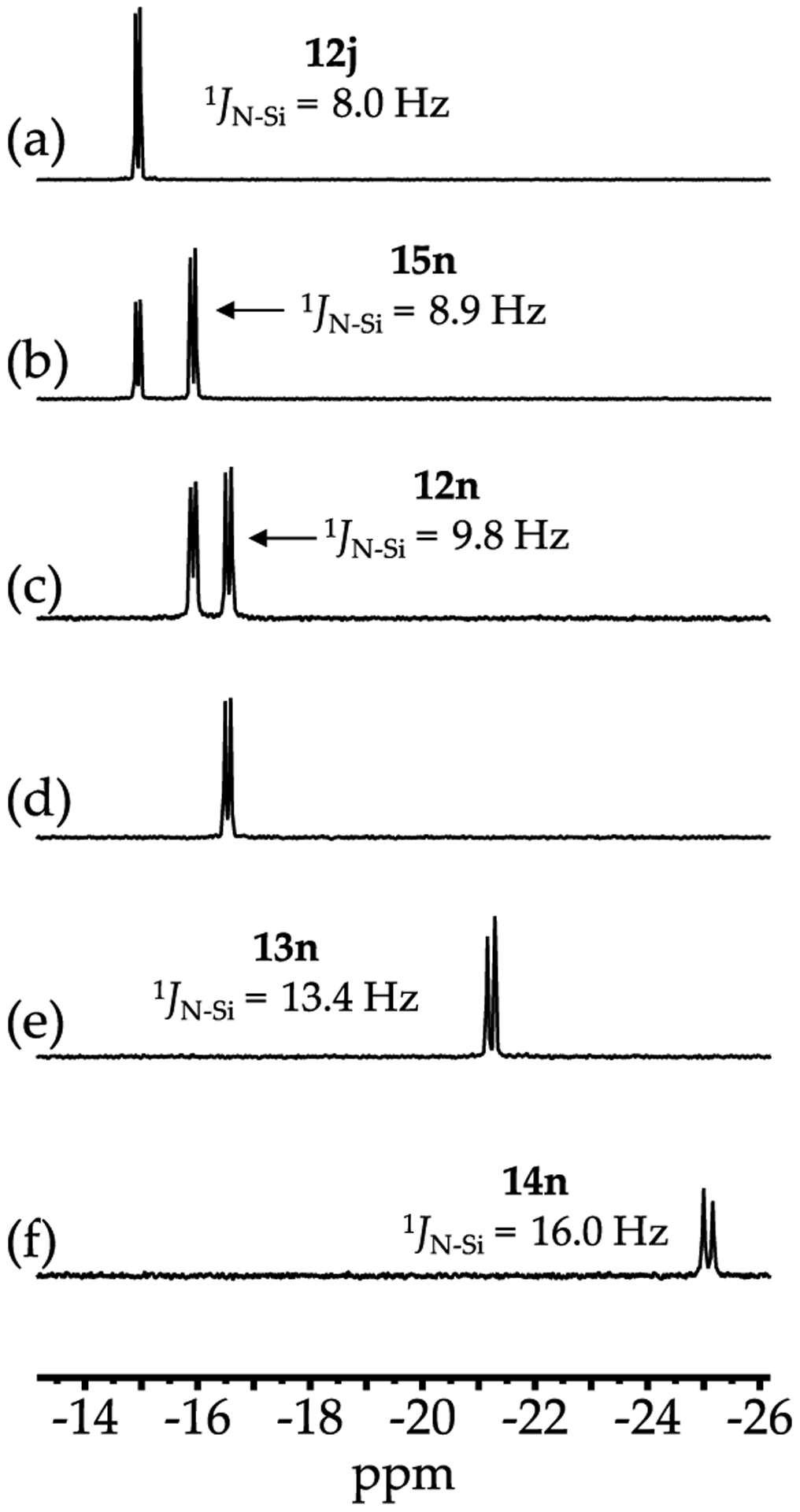
29Si NMR (various solvents, 99.36 MHz) spectra as follows: (a) (d) 0.19 M NaHMDS in 2:1 pentane/toluene-d8 at 120 °C with 0.0 equiv, 0.25 equiv, 0.75 equiv, and 1.0 equiv of HMPA, respectively; (e) 0.10 M NaHMDS in DMEA at 120 °C with 5.0 equiv of HMPA; and (g) 0.10 M NaHMDS in 2:1 HMPA/MTBE recorded at +40 °C (to resolve the coupling).
15N 29Si Scalar Coupling.
Figure 5 illustrates coupling that played an unexpectedly important role in the study. In the 1980s, Lukevics and co-workers reported 15N 29Si coupling constants for various disilazanes and several salts.9 Without the benefits of additional data, they attributed the 7.8 Hz 15N 29Si coupling for NaHMDS in benzene to a ligand-free tetramer, which we are now confident is the benzene-solvated dimer. We find that the 15N 29Si coupling constants correlate with the aggregation state: [15N]NaHMDS-containing homo- and heterodimers display 1JN Si = 7 9 Hz, whereas monomers display 1JN Si = 12 14 Hz (Table 1). The HMPA-solvated ion pair (Table 1, entry m) showed 1JN Si = 16.4 Hz, placing it outside the range of monomers.
The 15N 29Si coupling has the desirable feature that it can be monitored at any temperature, provided that the resonances are not in the midst of coalescing. Figure 6, for example, shows THF-concentration-dependent averaged couplings and 29Si chemical shifts affiliated with NaHMDS deaggregation. The fits attest to the relative solvation numbers of the dimer and monomer. Figure 7 compares the solvent-concentration-dependent coupling for THF and dioxane. The muted tendency of dioxane to deaggregate NaHMDS is evidenced by the intermediate coupling in neat dioxane, indicating ≈50 of the titer corresponds to the dimer.
Figure 6.
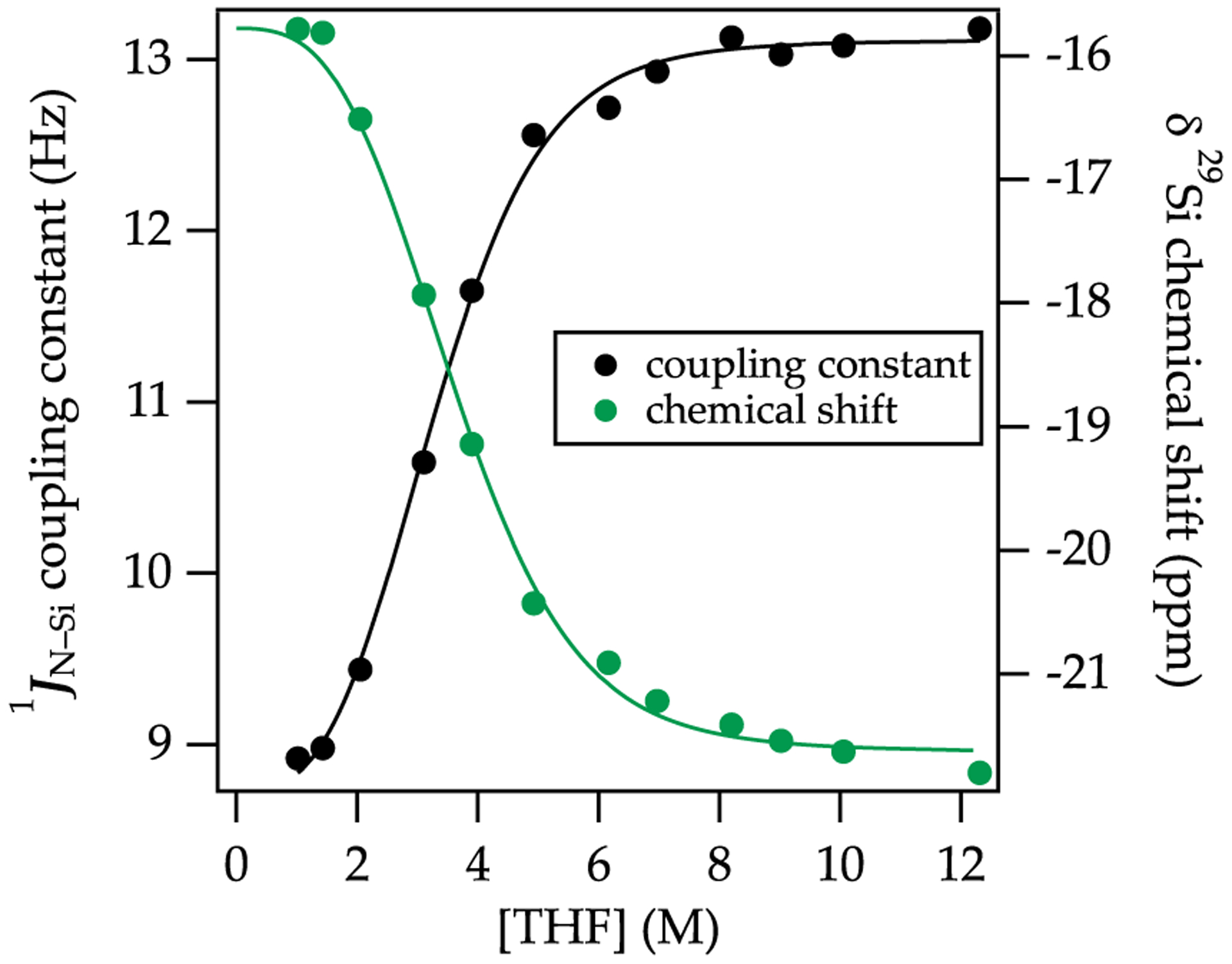
29Si chemical shift (green) and 15N 29Si coupling constants (black) plotted versus [THF] in 2:1 pentane/toluene as cosolvent measured at 20 °C. The functions are fit to a model based on an A2S2 AS4 equilibrium (Supporting Information). Reprinted from Woltornist, R. A.; Collum, D. B. Using 15N 29Si Scalar Coupling to Determine Aggregation and Solvation States. J. Am. Chem. Soc. 2020, 142, 6852. Copyright 2020 American Chemical Society.
Figure 7.
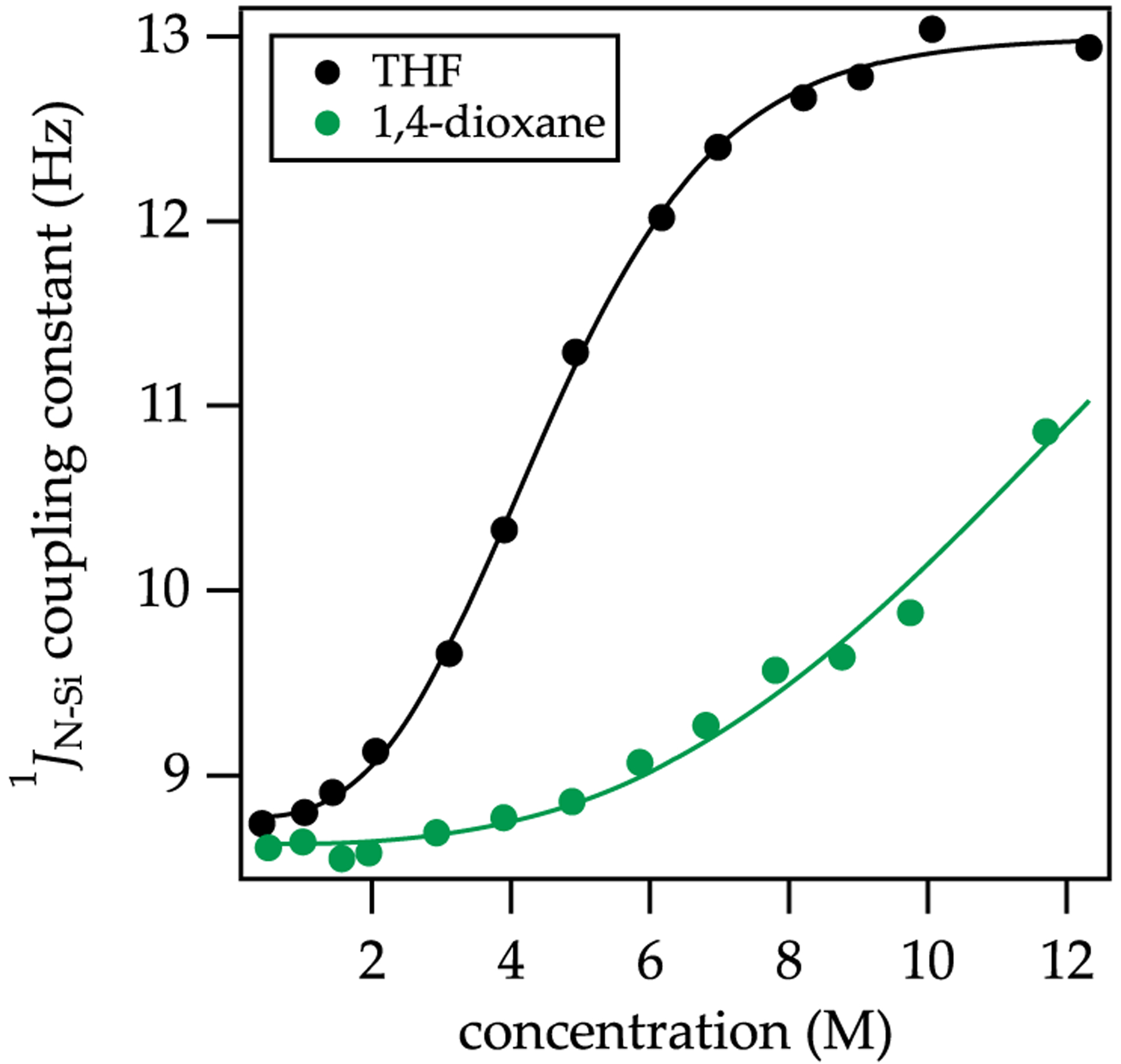
15N 29Si coupling constants plotted versus [THF] (black) and [1,4-dioxane] (green) in 2:1 pentane/toluene as cosolvent at 20 °C. The functions stem from a model based on an A2S2 AS4 equilibrium (Supporting Information). Reprinted from Woltornist, R. A.; Collum, D. B. Using 15N 29Si Scalar Coupling to Determine Aggregation and Solvation States. J. Am. Chem. Soc. 2020, 142, 6852. Copyright 2020 American Chemical Society.
We habitually use [15N]NaHMDS for all spectroscopic studies as a cross check. The per-sample cost of the label deriving from [15N]NH4C1 is approximately 7 the price of the NMR tube in which the spectra are recorded.
Titrations.
Solvation was studied by titration methods that were recently used for NaDA3 but have roots in the 1960s alkali metal literature.35 Serial additions of a coordinating solvent to NaHMDS in toluene elicit solvent-concentration-dependent 1H and 29Si chemical shifts of NaHMDS owing to ligand substitution. Figure 8 shows the binding of N,N,N ,N″,N″-pentamethyldiethylenetriamine (PMDTA). The linearity and sharp end point at 1.0 equiv of PMDTA per sodium attest to the quantitative binding of a single PMDTA per sodium ion. (The structure was subsequently shown to be a monomer as discussed below.) By contrast, weakly coordinating solvents such as Et3N do not quantitatively displace toluene from the NaHMDS dimer, as evidenced by curvature in the titration (Figure 9). In principle, the details of the curvature attest to the per-sodium solvation number, but such a distinction was not possible. Because of this and the slow exchanges observed at 120 °C, titrations played minor roles. They did, however, serve as an expedient method to monitor the solvent-concentration-dependent structural changes with serial additions through septa (Figure 5).
Figure 8.
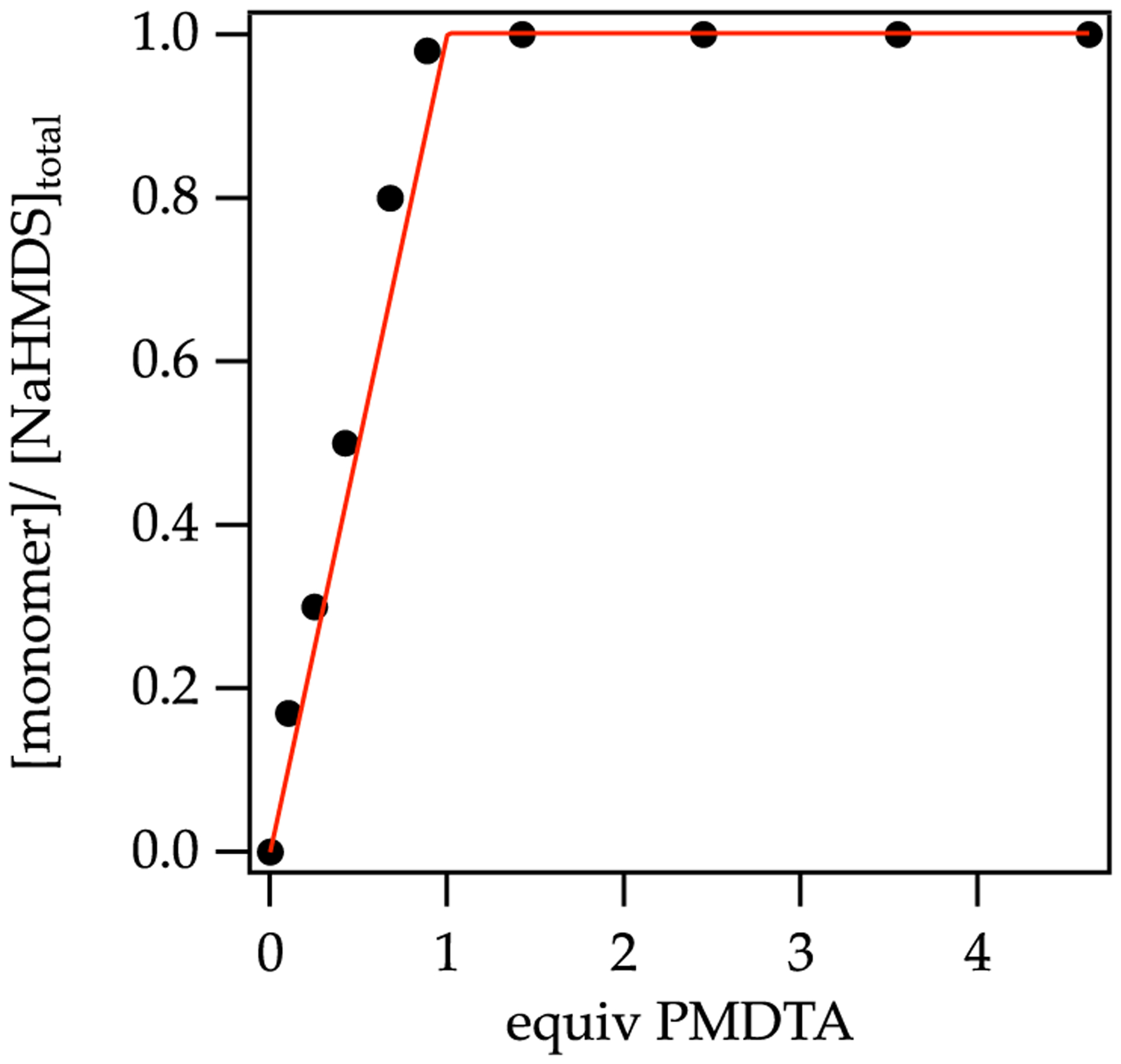
Plot of percent monomer versus equivalents of PMDTA in toluene at 80 °C.
Figure 9.
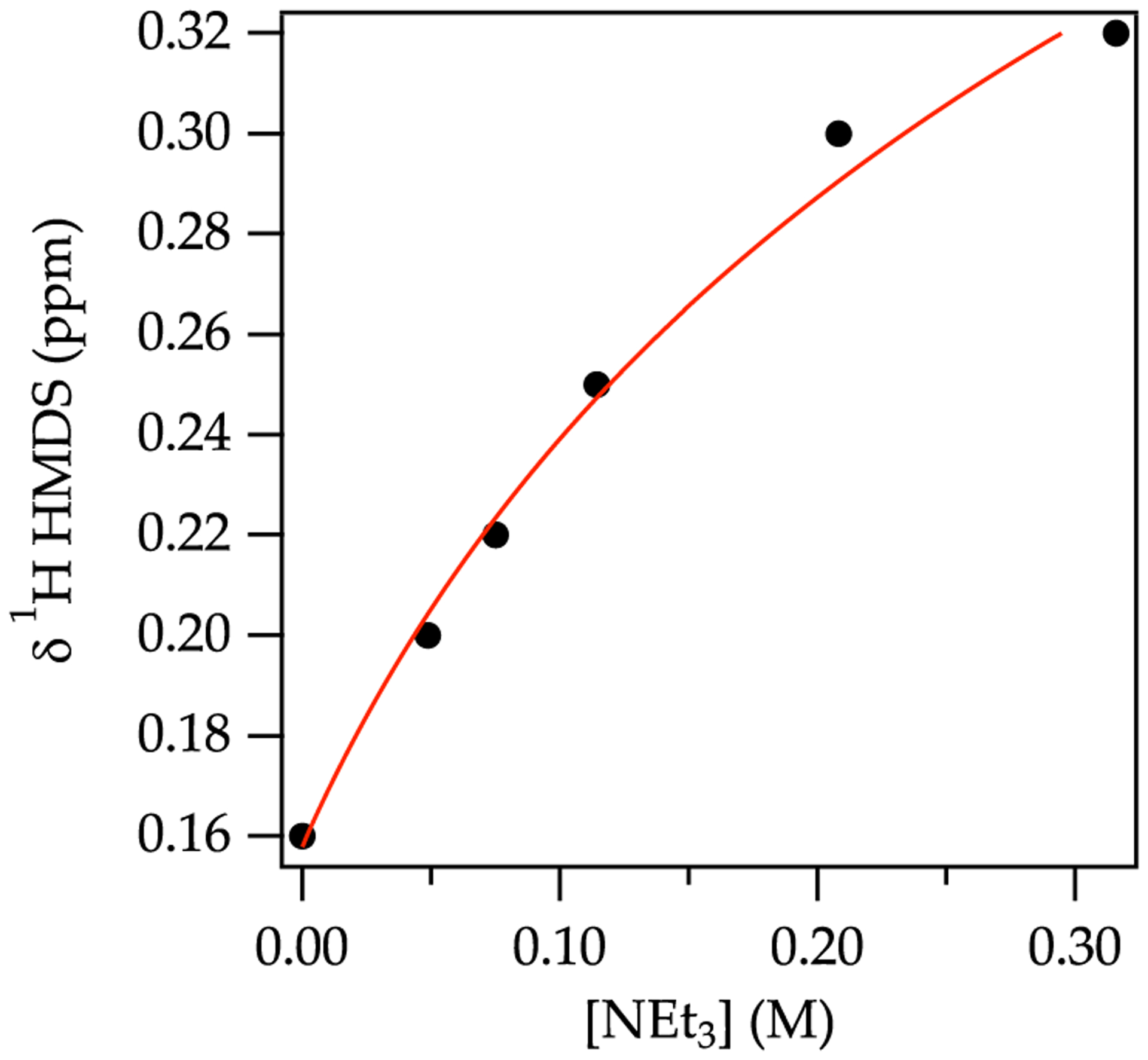
Plot of 1H chemical shifts of NaHMDS versus concentration of Et3N in neat toluene at 80 °C.36
Solvation in the Slow Exchange Limit.
In many cases, homosolvated and mixed-solvated dimers as well as chelated monomers were observed in the limit of slow exchange of free and bound solvent, offering unique probes of solvation numbers and correlated solvation (vide infra). Previous studies of LiHMDS show that slow exchange of monodentate solvents requires the rare combination of high barriers to both associative and dissociative ligand substitutions.14,37 This slow exchange manifests magnetically distinct 29Si signals for each form as illustrated in the serial titration of NaHMDS with HMPA (Scheme 1 and Figure 5). In some instances, separate 13C signals for free and NaHMDS-bound solvents are observable.
Computed Solvation Numbers.
The solvation numbers in Table 1 are derived from both experimental and computaional data. The free energies are reported on a solvent-per-sodium basis and benchmarked to their respective unsolvated dimers and monomers (eqs 2 and 3). However, there are
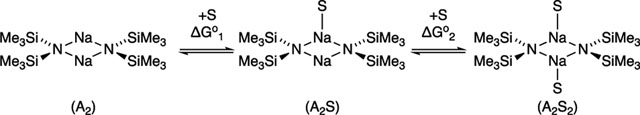 |
(2) |
deviations from nonstatistical behavior so-called “correlated solvation”38,41 in which G1 ≠ G2 and G3 ≠ G4 are prevalent and highly solvent dependent. The hindered bidentate ligand TMEDA, for example, shows G1 is markedly more negative (favorable) than G2 (eq 2),39 and G3 is more negative than G4 (eq 3). Similarly, where two or more solvents share a common sodium ion such as AS2 (eq 3), serial solvation is often correlated: G3 is more negative than G4. Despite such high correlations, Table 1 lists average free energies for the multiple solvent sodium interactions. The separate values for each solvation event are presented in the Supporting Information. Note that the reported free energies in Table 1 do not account for denticities. Chelated TMEDA has two sodium solvent contacts but is considered a single solvent. Thus, GTMEDA is probably most appropriately compared to 2 GTHF.42Acutely nonisodesmic43 comparisons
 |
(3) |
 |
(4) |
of dimers and monomers (eq 4) are less compelling and mentioned sparingly. Nonetheless, the solvation energies in Table 1, in conjunction with the energy of aggregation of the unsolvated dimer monomer (eq 5), enable the reader to calculate the solvent-dependent free energies of deaggregations for any solvent directly from the data in Table 1.
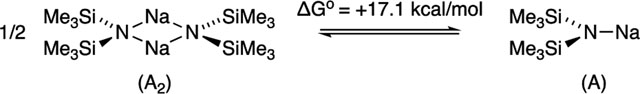 |
(5) |
Toluene and Other Hydrocarbons.
We now consider observations organized by solvent class with only limited comments about specific protocols. The solubility of NaHMDS in toluene and insolubility in saturated hydrocarbons foreshadowed the formation of an explicitly solvated dimer. Potassium hexamethyldisilazide and sodium triisopropyldisilazide crystallize as dimers bearing η6 toluene.44 Many η6 toluene sodium complexes45 and alkali metal arene complexes have been characterized crystallographically.32,34 Spectroscopic evidence suggests toluene binds, albeit weakly, to the LiHMDS dimer and ether- and amine-solvated monomers.14,15
Toluene-solvated dimer 12a was computed to be 0.3 kcal/mol/Na more stable than the crystallographically characterized trimer 1 (Chart 1). Computed serial solvation by toluene (eq 2) is moderately correlated: G01 = 2.7 kcal/mol and G02 = 0.5 kcal/mol (Figure 10). The solvation energy of the corresponding benzene solvate 12b shows the toluene methyl is not a major structural determinant. When titrating toluene solutions of NaHMDS by ethers and amines, one must account for the stabilization by two toluene ligands.
Figure 10.
DFT-computed toluene-complexed dimer 12a.
Previous studies showed that arenes, simple alkenes, and internal alkynes weakly bind to the LiHMDS dimer.14 A survey of potential nontraditional hydrocarbon solvents and cosolvents show that ethylene, propylene, 1-butene, 1-pentene, and 1,3-butadiene failed to measurably dissolve NaHMDS suspended in cyclopentane; the binding energies could be significant but are inadequate to override the lattice energy. Styrene dissolves NaHMDS, probably by forming computationally viable arene complex 12c, similar to those discussed in the context of anionic polymerizations.46
Methylene chloride could be useful and appears to interact with NaHMDS in toluene cosolvent at 78 °C, as evidenced by the change in the coupling constant (lJN Si = 8.4 Hz relative to lJN Si = 7.9 Hz in neat toluene). However, decomposition occurs within 1 h at 40 °C. NaTMDS, by contrast, is soluble and stable in CH2C12 at > 40 °C for >24 h. The relative reactivities of NaHMDS and NaTMDS support the notion that steric congestion increases reactivity through ground-state destabilization and underscores a potential niche for NaTMDS.
Monofunctional Solvents.
Monofunctional solvents fall into three approximate categories corresponding to weak, intermediate, and strong solvent donicities47 as determined by titrations, DFT computations, and inference from experience with LiHMDS and other lithium salts.14,16
Weak donors such as Et2O, MTBE, and trialkylamines (Table 1, entries d f, i, j) afford disolvated NaHMDS dimers to the exclusion of monomers even in neat donor solvent (eq 6). LiHMDS dimers solvated by sterically demanding and
 |
(6) |
weakly coordinating14,48 trialkylamines in toluene are beginning to find synthetic applications,15 as may NaHMDS R3N.49 The titration of NaHMDS/toluene solutions (Figure 9) shows nonquantitative displacement of toluene by Et3N (despite computations suggesting it should be quantitative). Although exchange of Et3N or DMEA was rapid at 120 °C, free and bound N-methylpyrrolidine was observed, confirming disolvation. The less congested disolvated NaTMDS homodimer 20 and NaHMDS NaTMDS heterodimer 21 (eq 1) show disolvation by Et3N in the slow-exchange limit. Computations clearly support disolvation as the norm for all trialkylamines. There is a steric limit, however: (i-Pr)2NEt (Hünig’s base) fails to solubilize NaHMDS in hexane, consistent with weak (transitory) solvation of LiHMDS.14
Solvents of intermediate donicity47 include THF, pyridine, pyrrolidine, 1,4-dioxane, and 1,3-dioxolane (eq 7). They are characterized by forming dimers at low solvent concentrations suggested by titrations and computations to be disolvates. Exchanges of free and dimer-bound solvents are fast at 120
 |
(7) |
°C and at low (<1.0 equiv) levels, wherein associative substitutions would be suppressed. Even moderately elevated solvent concentrations (>0.6 M) a ord monomers for THF, pyridine, and pyrrolidine. NaHMDS in THF (Table 1, entry 1) is arguably the most important structural assignment based on usage,4 prompting some comments and comparisons (Figure 11). THF-solvated dimer 12m is isostructural to the disolvated LiHMDS and LDA dimers.14,16 The THF-solvated NaDA dimer, by contrast, is tetrasolvated.3 THF-solvated monomer 13m is the sole observable form at >5 equiv of THF per sodium at 120 °C. Tetrasolvation is supported both experimentally (Figure 6) and computationally. A mixture of tri- and tetrasolvated monomers were implicated for the LiHMDS monomer.14
Figure 11.
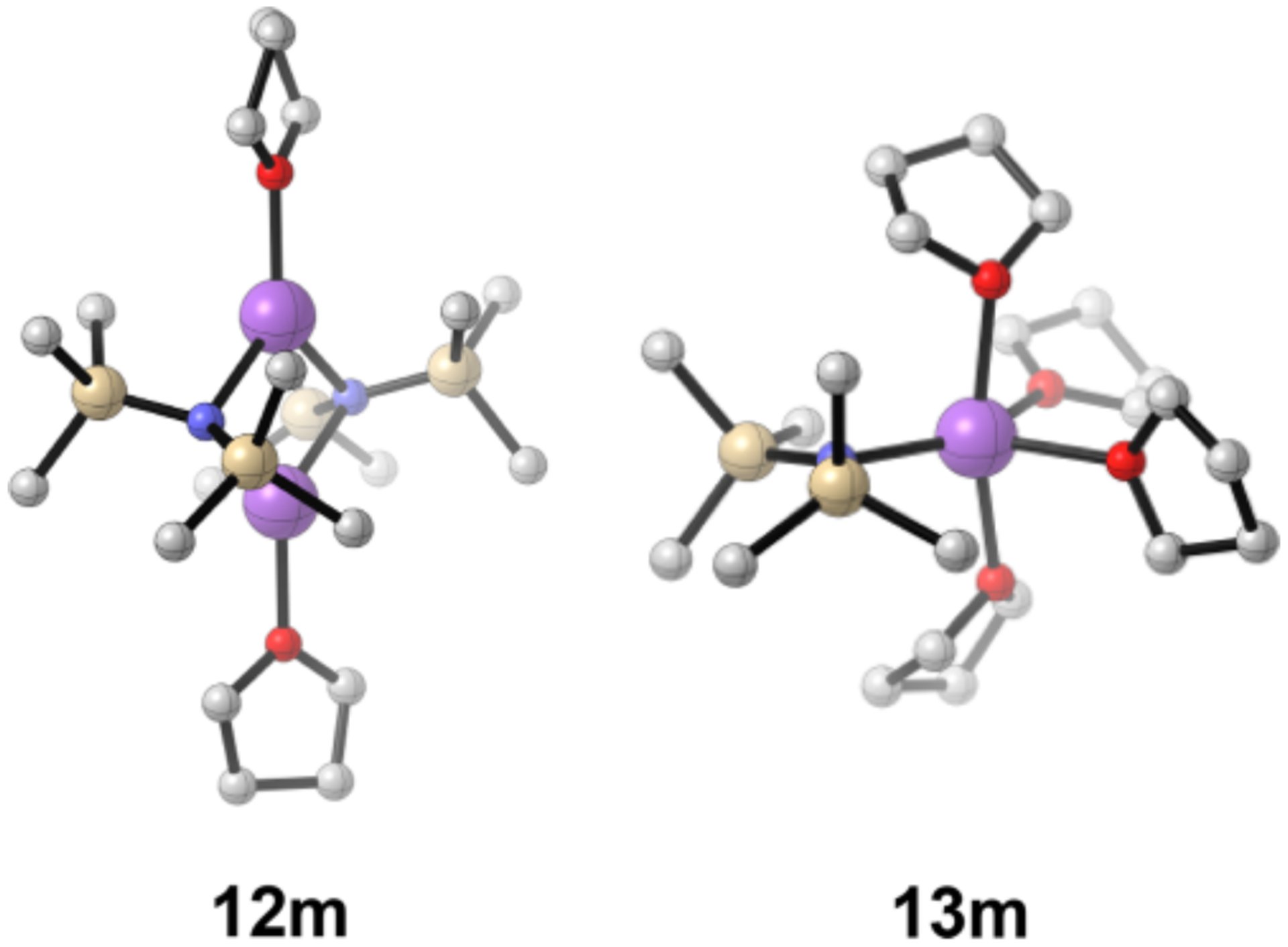
THF-solvated dimer 12m and monomer 13m.
Although dioxane is technically a difunctional ligand, weak binding and the reluctance to form monomers even in neat dioxane (Figure 7) indicate it is serving as a monofunctional ligand with a lower penchant than THF to deaggregate NaHMDS. We hasten to add that the approximately 6 kcal/mol of torsional strain in the boat form of dioxane50 accounts for why the chelated form appears to be without precedent. We witnessed gelling at low dioxane concentration even in MTBE, presumably due to linking NaHMDS dimer subunits into networks as found in monomer 9 (Chart 1).6h
Pyridine is shown both spectroscopically and computationally to be moderately superior to THF as a ligand for LiHMDS and other lithium salts.14 It may find niche applications in the chemistry of NaHMDS, but a potentially greater motivation would be to understand pyridine sodium interactions in putative51 SNAr reactions and other organosodium reactions of pyridine-based heterocycles.52 Both titrations and computations suggest the dimer is disolvated. Deaggregation by pyridine is detectably more pronounced than when using THF and is computationally suggested to be a tetrasolvated monomer. Lastly, although pyridine serves as a useful chemical shift reagent for 6Li NMR spectroscopy, we observe no notable 13C, 15N, or 29Si shifts in [15N]NaHMDS.
Pyrrolidine is representative of protic dialkylamines and is isostructural to THF. It is generally considered to be much more strongly Lewis basic than THF53 and tetrahedral at nitrogen akin to the oxygen of THF bound to sodium.54 Oddly, THF and pyrrolidine were indistinguishable as ligands for LiHMDS dimer, although pyrrolidine promoted deaggregation. Titration of NaHMDS with pyrrolidine (Table 1, entry h) shows a measurably more pronounced deaggregation for pyrrolidine when compared with THF, favoring a tetrasolvated monomer.
Monoalkylamines have the trappings of unhindered, highly Lewis basic solvents playing important roles in Birch reductions, SNAr reactions of halogenated arenes and heteroarenes, ester aminolyzes, and trans-amidations. The monoalkylamines unencumbered by additional alkyl groups are exemplified by n-BuNH2. Unfortunately, even at low concentrations of amine (<2.0 equiv per Na) in MTBE a coalescence is observed using 29Si NMR spectroscopy, most likely due to rapid exchange of multiple species.
The attenuated basicity of NaHMDS allows us to investigate strongly coordinating dipolar solvents that would otherwise be ravaged by NaDA and other organosodiums. Hexamethyl-phosphoramide (HMPA) offered the best view. Titration of NaHMDS in MTBE with HMPA shows serial formation of mixed and disolvated dimers, a monomer, and an ion pair monomer (Figure 5). At ≥1.0 equiv of HMPA monomer, 13n displays an HMPA-concentration-dependent chemical shift up to 3.0 equiv, at which point no further changes are noted. The stoichiometry and computations suggest that trisolvated monomer 13n (n = 3) is the limiting structure. A high amount (>15 equiv) of HMPA and elevated temperature (40 °C) afford a new species, manifesting an unusually large 16.4 Hz 15N 29Si coupling that we attribute to ion pair 14n. This is the only well-resolved 29Si doublet for such an ion pair.
31P NMR spectroscopy showed a single time-averaged resonance for the mono- and disolvated dimers and various solvated monomers. Broadening occurs at 1.0 2.0 equiv/Na, which decoalesces at 5.0 equiv of HMPA to show free HMPA and two mounds in an approximate >10:1 ratio. Monomer 13n and ion pair 14n are logical candidates.
Analogous titrations of NaHMDS in MTBE with DMPU serially solvate through 15o and 12o. Higher DMPU concentrations cause the 29Si resonance to disappear; we suspect the formation of ion pair 14o.
Looking for dipolar solvents without the stigma of HMPA, we turned toward DMSO and DMF. Titration of NaHMDS in DMEA with DMSO at 80 °C resulted in a single 29Si resonance with a DMSO-concentration-dependent chemical shift and coupling constant signifying a change from dimer to monomer. Even at low DMSO concentration, the monomer was the dominant species. However, at >3.0 equiv, a coalescence was observed. DMF performed poorly, resulting in only dimer and low concentrations of monomer at >2.0 equiv with evidence of decomposition.
Difunctional Solvents.
Difunctional ligands were not as predictable as expected. The low steric demands of DME (compared with TMEDA),14 for example, seemed likely to support doubly chelated dimer, and indeed, computations show strong binding and only limited correlated solvation. Titration of NaHMDS in DMEA with DME (Table 1, entry u) at 120 °C, however, showed a DME-solvated monomer and DMEA-solvated dimer 12d concurrently at <2.0 equiv of DME, evident from an unchanged dimer coupling constant consistent with 12d. We suspect cooperative solvation is at play here, giving rise to a DMEA DME heterosolvated monomer at these low DME concentrations. Monomer 13v is the sole observable form at ≥3.0 equiv. TMEDA is the quintessential bifunctional ligand that has been instrumental in shaping the thinking of researchers for generations about structure reactivity relationships for alkali metal chemistry (eq 8).55
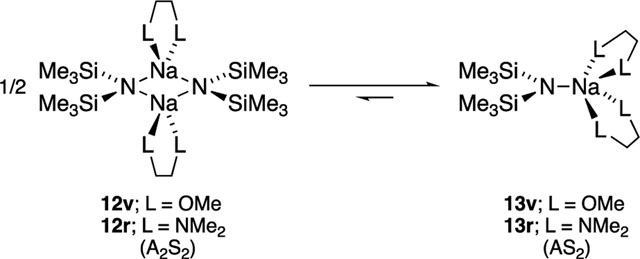 |
(8) |
NaHMDS TMEDA in DMEA shows a mixed-solvated dimer at <1.0 equiv and a hard end point at 1.0 equiv per sodium, which, in conjunction with MCV and 15N 29Si coupling data, is consistent with chelated dimer 12r (Chart 2). Two equiv of TMEDA affords equal proportions of free and bound TMEDA in the slow exchange limit (≤ 120 °C). Decoalescence of the methylene resonances in the TMEDA backbone (but not the methyls) evidences a half-chair conformer56 exchange. Computed weakening of the second TMEDA ligand (highly correlated solvation) is affiliated with one of the four TMEDA sodium contacts being elongated (Figure 11). This may be merely a computational overestimation of steric effects, which we have witnessed on many occasions.57 Addition of >0.80 M (>5.0 equiv) of TMEDA forms a monomer. Five-coordinate κ2,κ2-13r is computationally viable, but four-coordinate κ1,κ2-13r is 2.0 kcal/mol more stable (Figure 12).
Figure 12.
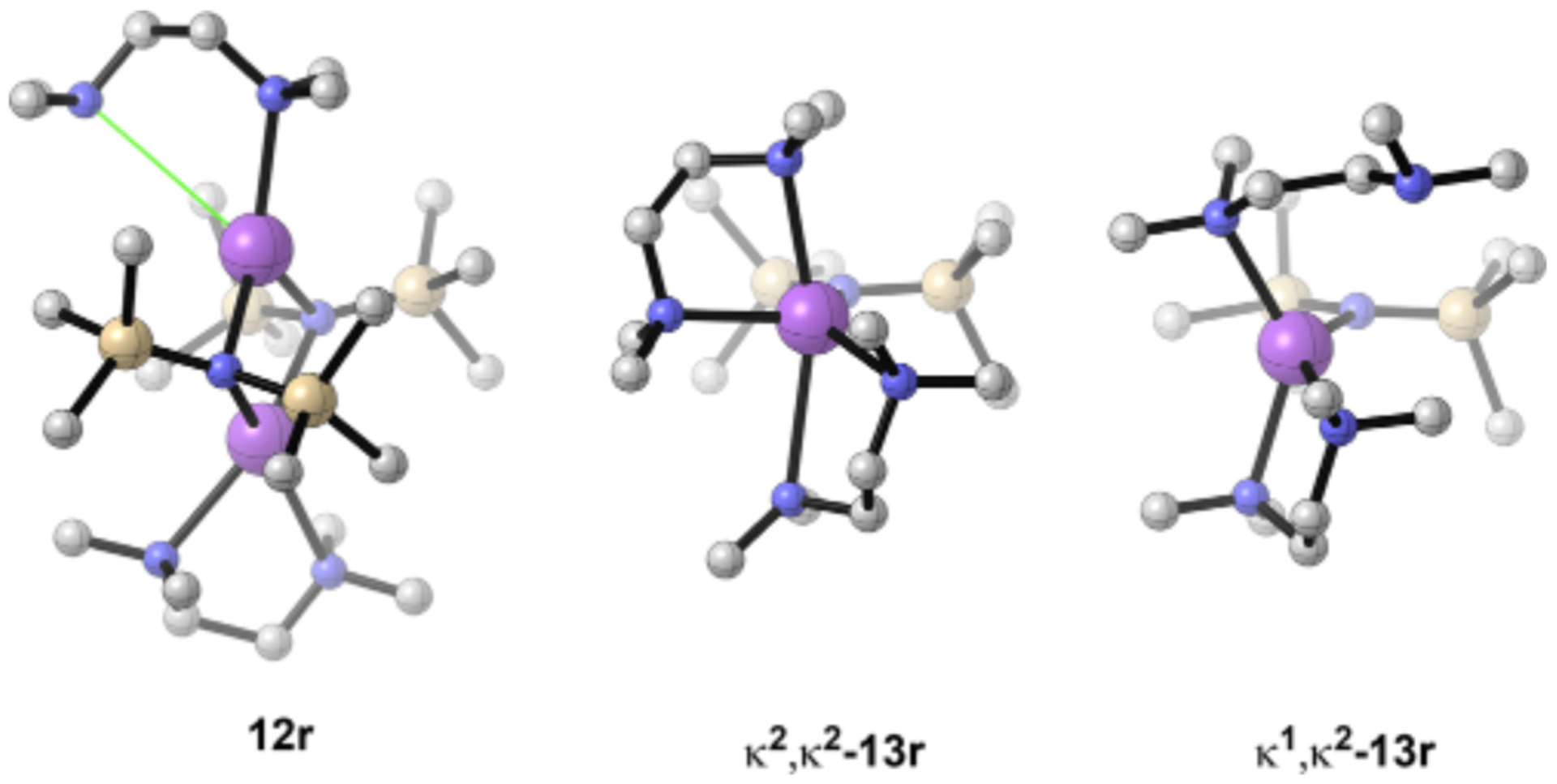
DFT-computed structures of TMEDA-solvated dimer 12r showing an elongated N Na contact and bischelated monomer 13r.
Analysis of the time-averaged coupling constants for both DME and TMEDA at 20 °C (Figure 13) reveals a stark contrast in the solvation energies for these two ligands. While DME forms 12v at low concentrations (<0.50 M), TMEDA struggles to form monomer even in neat TMEDA. Overall, these results contrast with LiHMDS/DME, showing κ1-solvated dimers and chelated monomers,14 LDA showing only κ1-solvated dimers,16 and NaDA showing only κ2-solvated dimers.3
Figure 13.
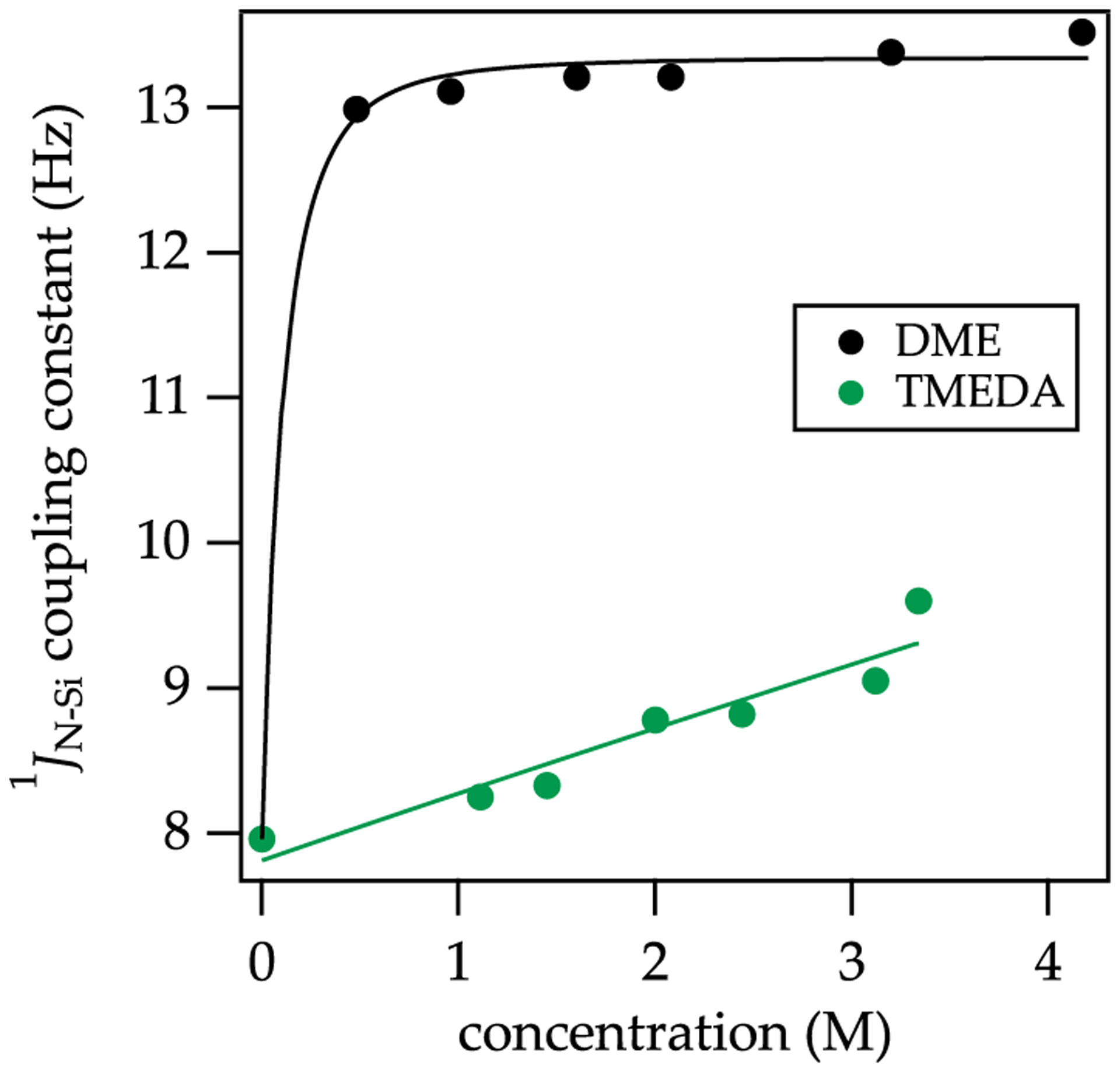
15N 29Si coupling constants plotted versus [DME] (black) and [TMEDA] (green) in 2:1 pentane/toluene as cosolvent at 20 °C. The functions are fit to a model based on an A2S2 AS2 equilibria.
(R,R)-Tetramethylcyclohexanediamine (TMCDA) often serves as a strongly coordinating TMEDA surrogate in organolithium chemistry14,16 and has attracted some interest owing to its chirality. However, the same cannot be said about NaHMDS. A single new species displaying two (diastereotopic) 29Si resonances (1:1) with dimer-like coupling persists at >3.0 equiv of TMCDA. Analogous spectroscopic data showing two distinct silyl moieties prompted O Hara and co-workers6 to suggest crystallographically characterized 4 retains its structure in solution. Given the two distinct 29Si resonances and the excess of DMEA, mixed-solvated analogue 15s seems logical; however, a highly fluctional mixed-solvated open dimer of general structure 24s cannot be excluded. To ascertain whether TMCDA fails to bind owing to unforeseen steric effects such as rigidity or poor bite angle on the larger sodium ion,59 we compared TMEDA versus TMCDA for LiHMDS and NaHMDS monomer fragments (eq 9). Though
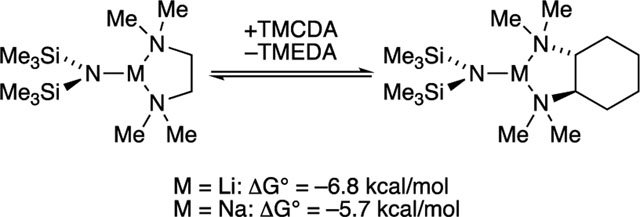 |
(9) |
both LiHMDS and NaHMDS prefer TMCDA-solvated monomer over TMEDA-solvated monomer, the relative binding energy comparing lithium and sodium qualitatively suggests an elevated monomer preference for lithium.
Sparteine (25) has been a workhorse chiral ligand in organolithium chemistry60 and has shown demonstrably strong binding to the LiHMDS monomer.14 By contrast, >3.0 equiv of sparteine shows no evidence of binding to NaHMDS in DMEA (Table 1, entry s). We suspect sparteine is too sterically demanding to chelate to dimers or doubly chelate monomers despite crystallographic evidence that less congested sodium salts can support two bound sparteines.61
The relatively low reactivity of NaHMDS has allowed us to probe the common transition metal ligand, 2,2 -bipyridine (bipy). At 0.50 equiv of bipy in DMEA, mono- and dichelated dimers are observed. We also detect what appears to be low concentrations of open dimer (26u). At 1.0 to >3.0 equiv of bipy, a disolvated dimer persists to the exclusion of monomers. No spectroscopic evidence of destruction is observed after days at room temperature even though the solution color turns hot pink.
Protic amines often presented problems in studies of LiHMDS owing to facile exchanges, causing loss of 6Li 15N coupling. We wondered if we would have anticipated the merits of 15N 29Si coupling if the story had been different. Titrations of NaHMDS with ethylene diamine led to chronic solubility problems even using THF as the cosolvent, possibly due to intervening formation of ion pair 14 or extended hydrogen-bonded networks.62
N,N,N ,N″,N″-Nonamethylimidodiphosphoramide (NIPA, 27)63 is highly dipolar, potentially chelating, and possibly an HMPA surrogate, albeit with unknown toxicity. Unpublished work showed that NIPA a ords exclusively monomeric LiHMDS.64 NIPA has been evaluated as a ligand for a number of inorganic metal salts65 but has been almost totally overlooked by the alkali metal community.66,67 Although the preference of five- versus six-membered ring chelates for lithium is fully established,14 the preference for sodium is less obvious, largely from a lack of detailed, systematic studies.68 Unfortunately, titrations of NaHMDS with NIPA resulted in a mixture of species at ≤1.0 equiv and precipitation above 1.0 equiv: NIPA may find niches in time.
Trifunctional Solvents.
We have ongoing studies of PMDTA on other sodium salts and believe it will be a ligand of central importance to the further development of organosodium chemistry; crystallographers have already discovered its merits.1d,10 Titrations show exclusively NaHMDS monomer (eq 10) with free and bound PMDTA in slow exchange in pentane/toluene at 100 °C. NaHMDS-bound PMDTA displays seven resonances of equal intensity and two resonances corresponding to the two sets of time-averaged terminal methyl groups. Although κ2-PMDTA-solvated dimer 12w could afford eight carbons, MCV and large 15N 29Si coupling confirm the monomer assignment. The magnetic inequivalency of the PMDTA methylenes at low temperature
 |
(10) |
and coalescence to give seven resonances of the bound form at elevated temperature is consistent with half-chair conformers observed for lithium complexes of PMDTA.14,69 DFT computations support the high preference for monomer 13w relative to doubly chelated dimer 12w and showed three distinct monomer conformers, of which the conformer in Figure 14 is preferred.
Figure 14.
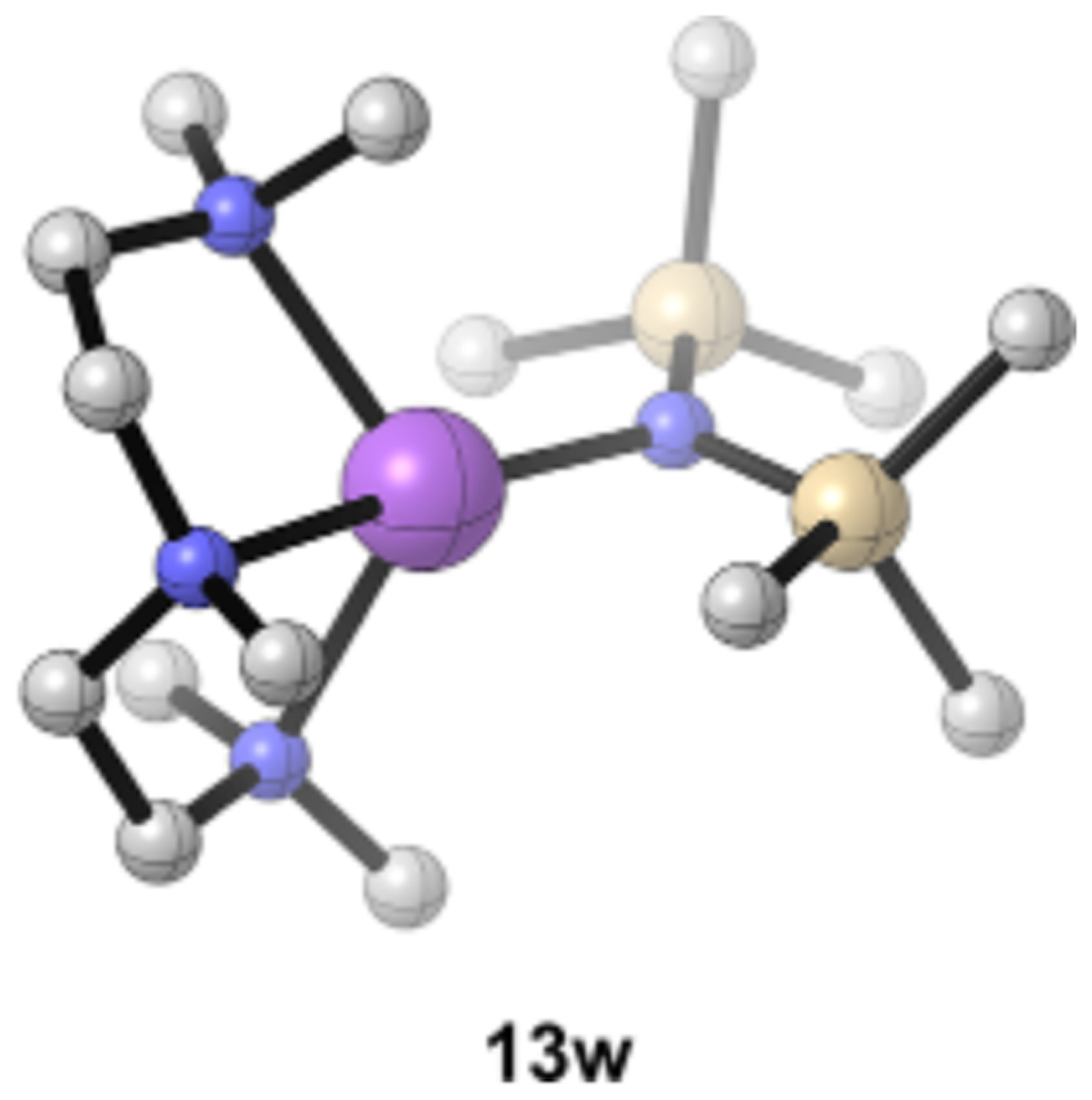
DFT-computed lowest-energy conformer of PMDTA-complexed monomer 13w.
Diglyme is labile to strong bases but not to NaHMDS, and it is extremely cost-effective for applications in synthesis. NaHMDS with ≥1.0 equiv of diglyme shows exclusively monomer 13x (eq 10) with free and bound diglyme time averaged at 120 °C. Phase separation or peak broadening emblematic of ion pair formation was not observed. Diethylenetriamine, an unhindered analogue of PMDTA and an isostructural analogue of diglyme, by contrast a orded intractable amorphous solid even in THF solution, possibly, owing to ion pair formation (see above) or hydrogen-bonded networks.62
Polyfunctional Solvents.
In 1996, we reported that LiHMDS monomer solvated by the three parent crowns 12-crown-4 (28), 15-crown-5 (29), and 18-crown-6 (30) showed two odd features that conflicted with consensus: (1) the three crowns display nearly the same binding constant (±0.5 kcal/mol) and (2) all three proved comparable to THF or TMEDA.14 It was in this context that we investigated the binding of 28 30 to NaHMDS (Scheme 2).
Scheme 2.
Crown Ether Complexes of NaHMDS
Adding the crown ethers to NaHMDS in DMEA caused phase separation of amorphous liquids or solids. On the positive side, 15-crown-5 afforded diffractable crystals of 13z, the first crystallographically characterized NaHMDS crown complex (Figure 15). However, due to poor crystal quality, only atom connectivity was ascertained.
Figure 15.
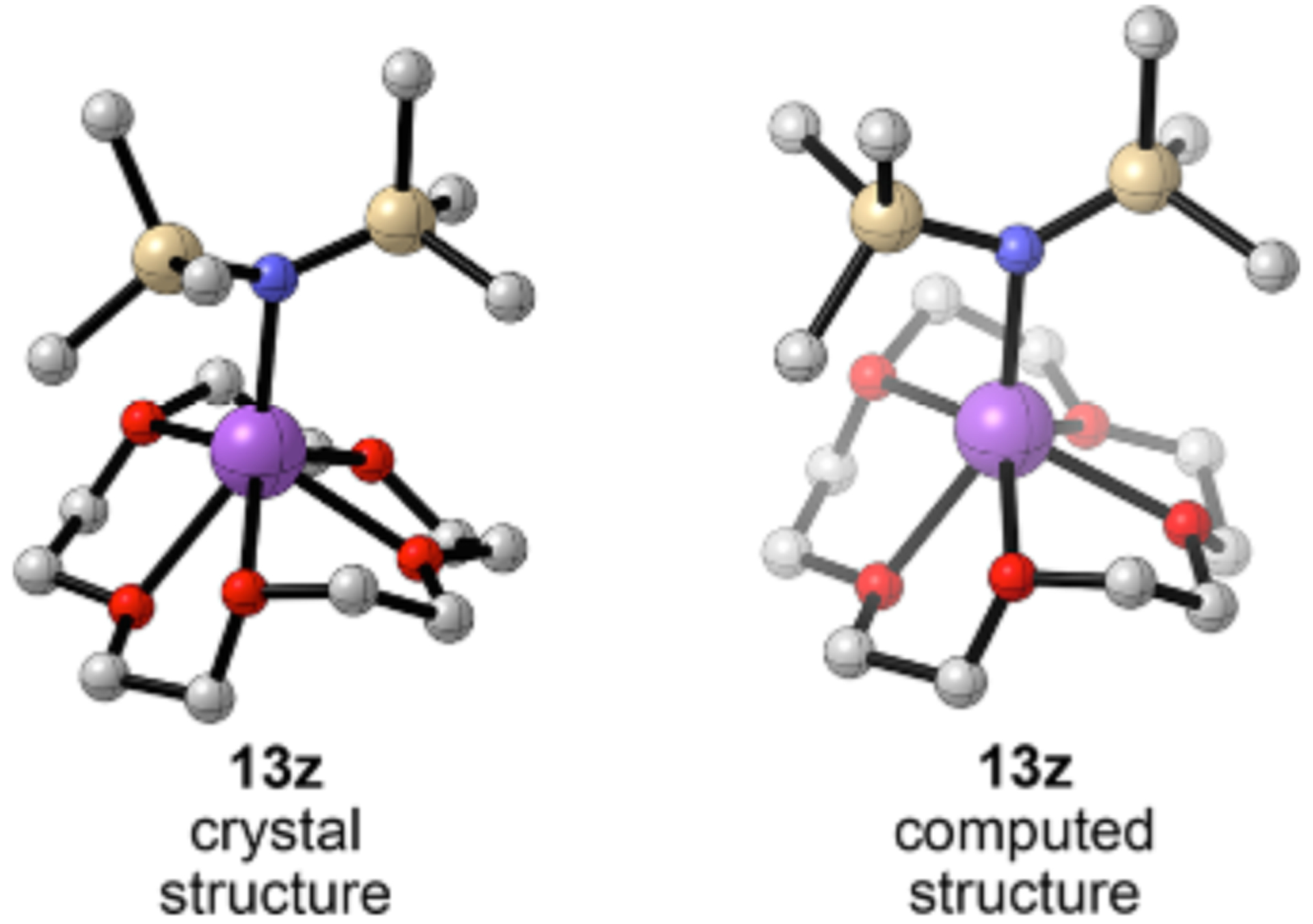
Crystal and computed structure of NaHMDS 15-crown-5 complex 13z. Arrows indicate the longest Na O contact.
Monomer 13z shows four Na O close contacts (2.44 2.59 Å) that mimic two DME ligands and an elongated (2.60 Å) fifth Na O interaction. DFT computations show similar structural features.
Titrating NaHMDS with crown ethers 28 30 in THF at 105 °C a orded homogeneous solutions with strong evidence of crowns remaining complexed. Titrations using NaHMDS in MTBE with 12-crown-4 converts MTBE-solvated dimer 12j to crown-complexed monomer 13y (1:1 stoichiometry) with no detectable intermediates. At approximately 1.5 equiv of 12-crown-4, a solid precipitates, consistent with ion pair 14y. By contrast, 0.50 equiv of 18-crown-6 consumes >95 of MTBE-solvated dimer 12j, affording a new species to the exclusion of other forms suggested by stoichiometry to be triple ion 16aa. At 1.0 equiv, 16aa is converted to exclusively monomer 13z. Excess crown a ords broad upfield 29Si resonances ascribed to ion pair 14aa. Titration with 15-crown-5 affords intermediate behavior. Triple ion 16z and monomer 13z are formed concurrently, with monomer 13z becoming the sole species at 1.0 equiv. At ≥1.0 equiv of 15-crown-5, monomer 13z crystallizes from solution. Crystallization is accelerated by warming also. Ion pair 14z might be viable but is precluded by the crystallization.
Free and NaHMDS-monomer-bound crowns are observed in slow exchange, revealing 1:1 NaHMDS:crown stoichiometries for 29 and 30 and magnetically equivalent carbons despite computations showing significant distortions (Figure 16). High fluctionality is likely the source of the apparent high symmetry.
Figure 16.
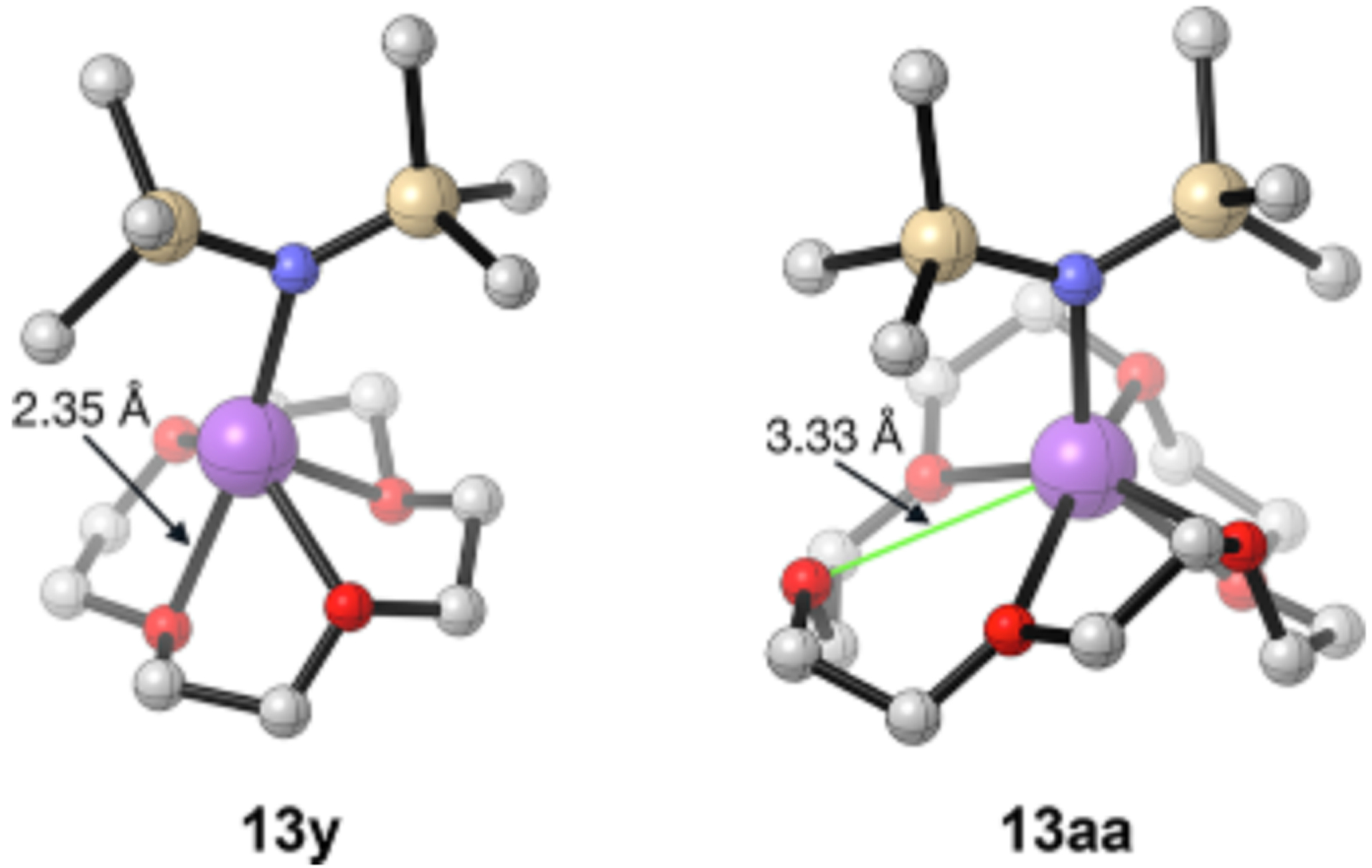
DFT-computed structures of crown-solvated monomers 13y and 13aa. Arrows designate typical Na O bond lengths in 13y and the longest Na O (unbound) contact in 13aa.
The evidence of triple ions 16z and 16aa initially was based on the sub stoichiometric quantities of crown required to consume MTBE-solvated dimer 12j. To confirm the assignments, a 1:1 mixture of [15N] NaHMDS/NaTMDS in MTBE showing statistical mixtures of homo- and heterodimers (eq 1) was titrated with 18-crown-6, a ording an ensemble of homo-, and heteroaggregated triple ions (l6aa and 30aa). Monomers 13z and 13aa were also confirmed by the absence of heteroassociation. The uniquely high preference for a triple ion with 18-crown-6 may be because only 18-crown-6 can fully * encapsulate the sodium within the crown (Figure 17),70 which also impacts the binding of the MTBE cosolvent.
Figure 17.
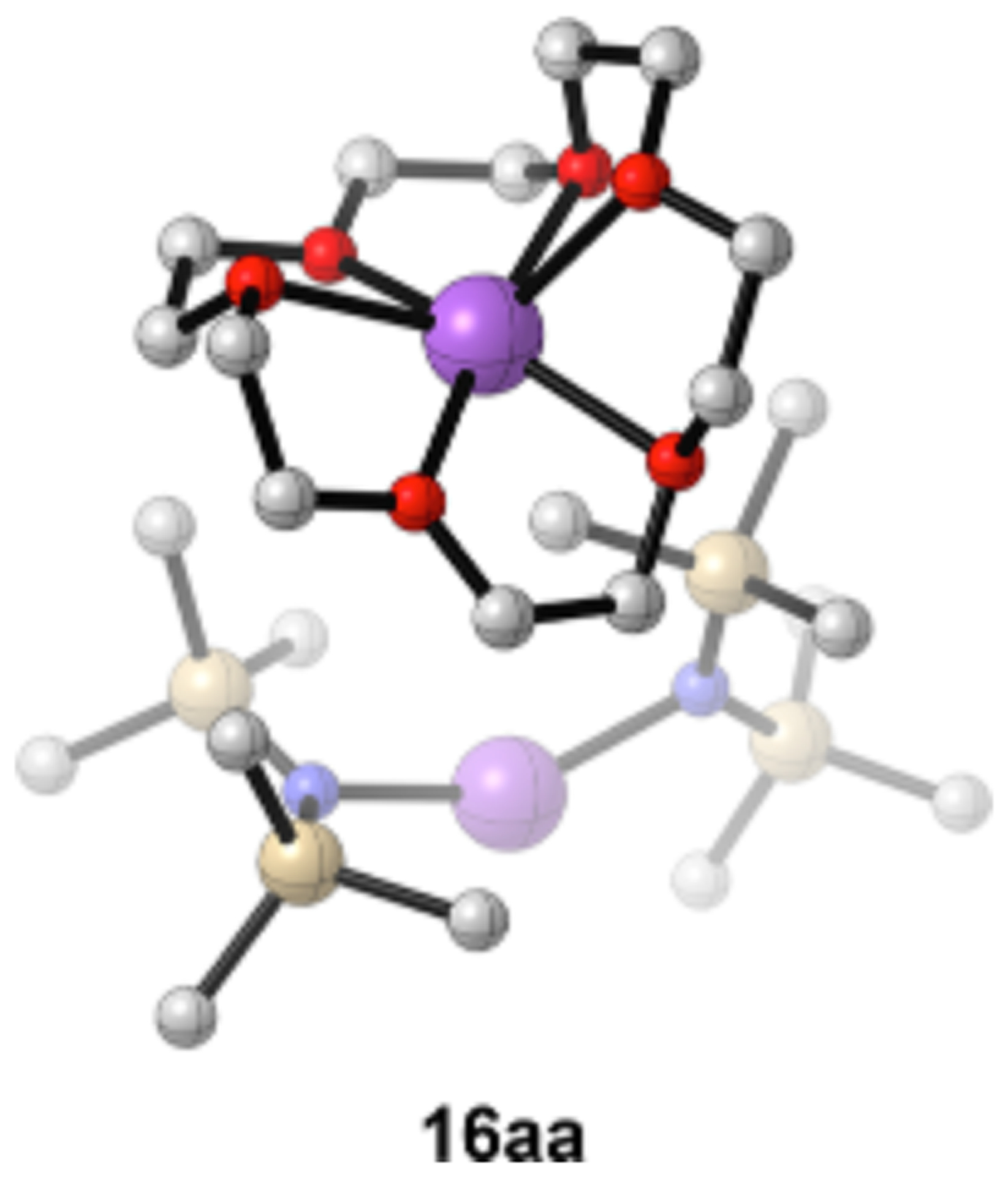
Computed structure of 18-crown-6-complexed triple ion 16aa.
The final step was to compete the crowns against each other to ascertain relative affinities for NaHMDS. Titrations of NaHMDS in MTBE with stock solutions containing 1:1 mixtures of two crowns by monitoring the resolved 29Si, resonances revealed nearly indistinguishable binding of the three crowns to monomer 3 (Scheme 3).71
Scheme 3.
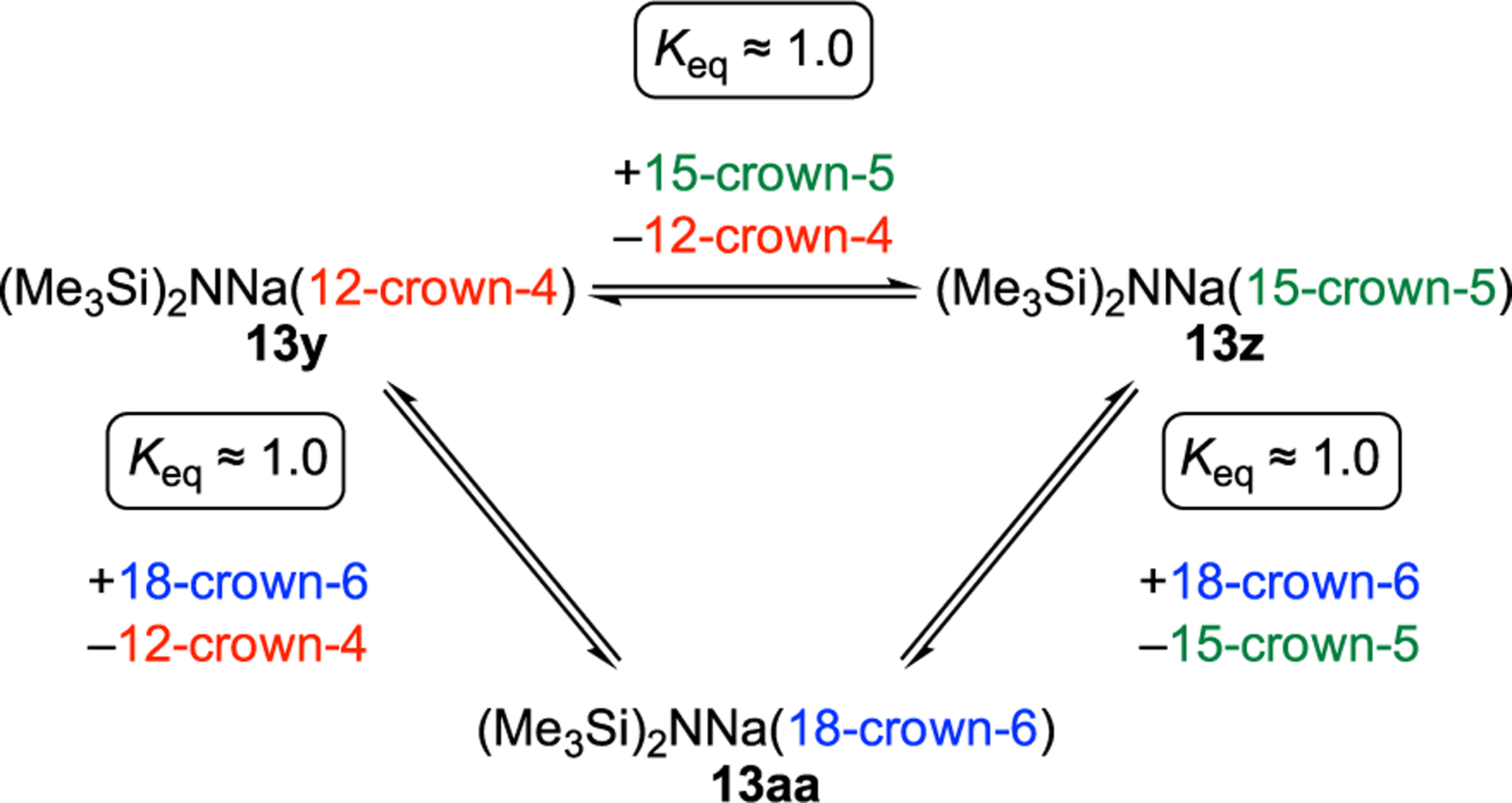
Competition of Crowns Showing Nearly Equal Binding to Form Monomers 13y, 13z, and 13aa
Polyether 31, referred to as TDA-1, has its roots in phase transfer catalysis.72 It displays cryptand-like behavior with LiHMDS.14 Serial titrations of NaHMDS a ord a triple ion at 50 equiv that was confirmed to show a heteroassociated form when mixed with NaTMDS. Monomer 13bb forms to the exclusion of ion pair 14bb at ≥1.0 equiv.
NaHMDS and cryptand [2.2.2] (32) in DMEA a ord a white crystalline solid. An X-ray crystal structure shows the anticipated cryptate shown in Figure 18. An analogous structure has been reported by Stephan et al. for the KHMDS-cryptand complex.73 Addition of 0.50 equiv of 32 to NaHMDS in THF causes the disappearance of monomer 13m and the appearance of triple-ion-based cryptate 16cc, along with low concentrations of ion pair 14cc as a broad mound.
Figure 18.
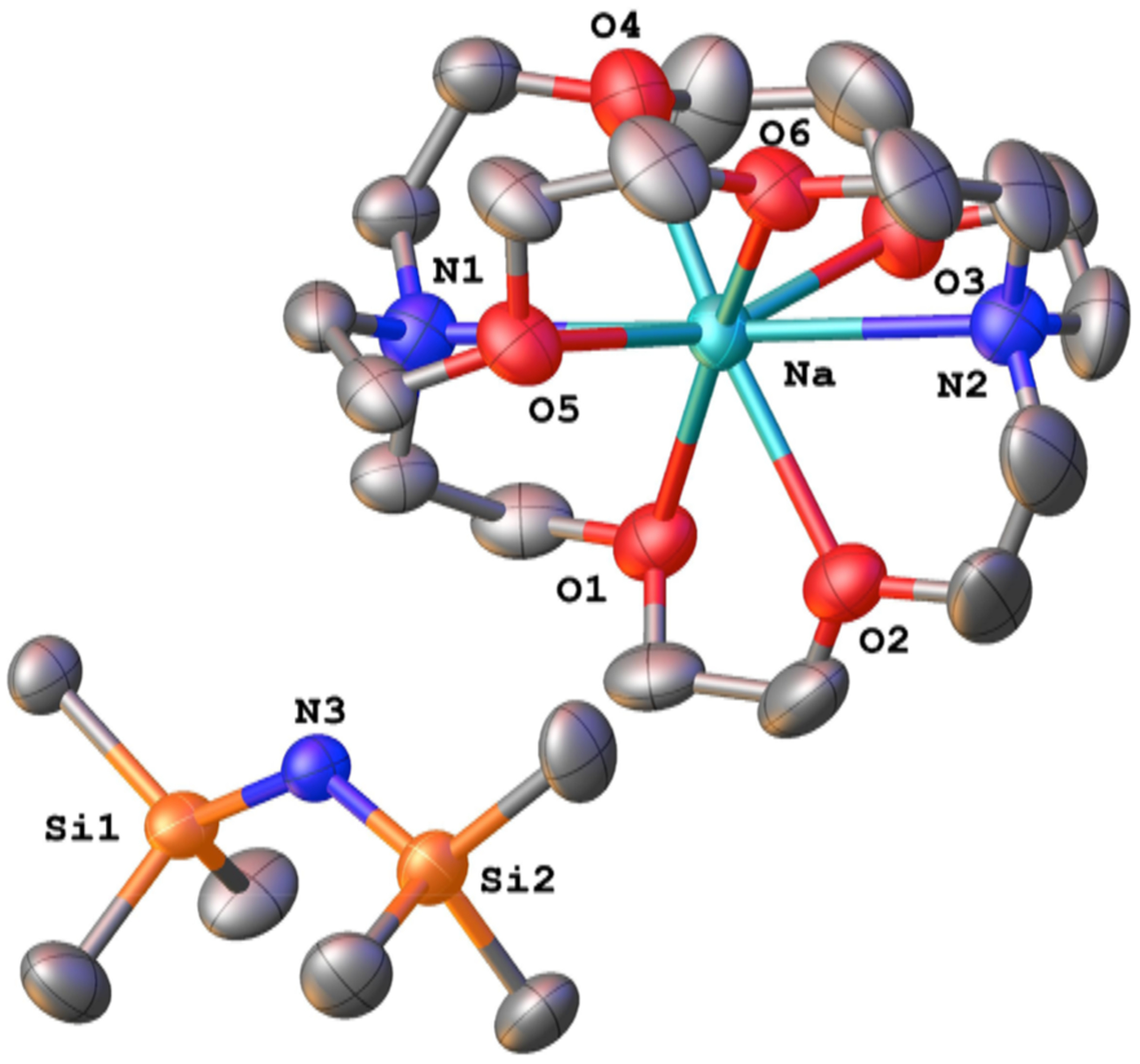
ORTEP drawing of 14cc formed from NaHMDS and cryptand 32 showing thermal ellipsoids at the 50 probability level.
Relative Solvation Capacities and Cooperative Solvation.
We have described much of what we learned about the capacity of various solvents to compete with each other. We are careful not to call it solvation energy per se because we are comparing different structural forms. With that said, the overall capacities of solvents to compete are summarized in Scheme 4, using the most important solvents within their respective classes. The weakly bound ethers and trialkylamines reluctantly substitute toluene on the NaHMDS dimer but are easily displaced by ligands designated as having intermediate donicity such as THF. All but the weakest ligands also readily afford monomers at elevated ligand concentrations. A study of diamines showed TMEDA to be far superior to TMCDA. Binding of the crown ethers to the NaHMDS monomer is shockingly independent of crown structure; this result was foreshadowed by studies of LiHMDS.14 There remained, however, a few questions central to our understanding that required explicit competitions not described above. In this context, PMDTA is a pivotal divide between moderately and strongly binding ligands and a useful benchmark.
Scheme 4.
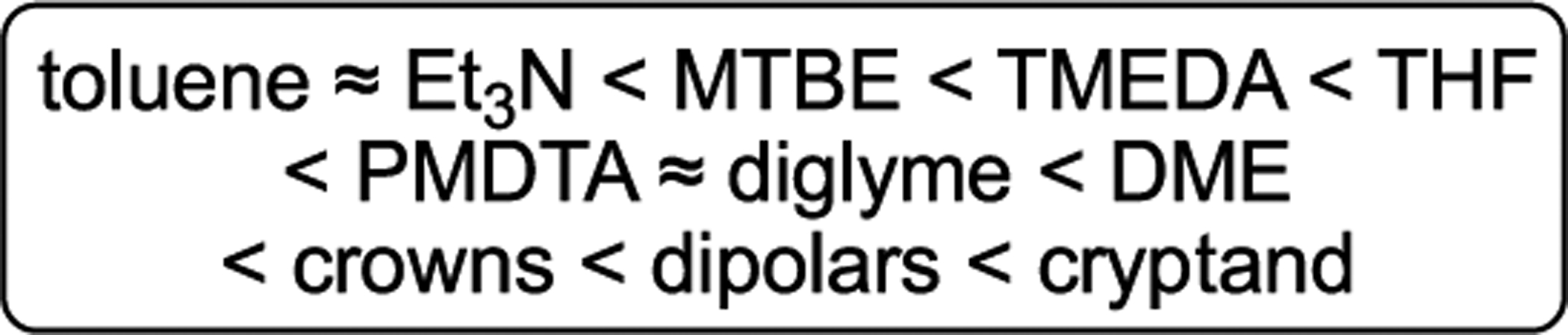
Scale of Relative Competitive Binding to NaHMDS
Competitions show that TMEDA cannot compete with THF, which was also documented for LiHMDS,14 and THF cannot compete with PMDTA for monomer solvation. THF and other monodentate donor solvents do, however, catalyze the exchange of free and bound PMDTA as evidenced by coalescence of the PMDTA resonances in the 13C NMR spectra.
Di-, tri-, and polyfunctional ethereal ligands versus PMDTA allow for the assignment of their relative binding affinities. Competing PMDTA and diglyme a ord a time-averaged 29Si signal, but the intermediate chemical shift suggests that 13w and 13x coexist. The most important comparison was, in our opinion, PMDTA and the crown ethers. In titration of NaHMDS with 1:1 stock solutions of PMDTA and 12-crown-4, the crown-solvated monomer is favored, suggesting that κ4-polydentate ether-based monomers are favored over the κ3-PMDTA-based monomer.71 Furthermore, competing PMDTA and DME showed a strong preference for DME-solvated monomer 13v. However, using this same titration method with DME and crown 28 resulted in only crown complex 13y at 1.0 equiv of each ligand. Continued addition of both ligands resulted in an increase of the 15N 29Si coupling constant and a decrease in signal intensity, indicating cooperative solvation of an ion pair.
One might surmise that the dipolar ligands bind more strongly than PMDTA and THF. Competitions of PMDTA and HMPA show HMPA solvate 13n to be the sole observable species. Curiously, equimolar PMDTA and HMPA at 1.0 equiv of total ligand concentration showed a new 29Si signal corresponding to low concentrations of triple ion, suggesting cooperative solvation is at play. It reminds us that combinations of two ligands may cooperatively provide access to atypical aggregation states.
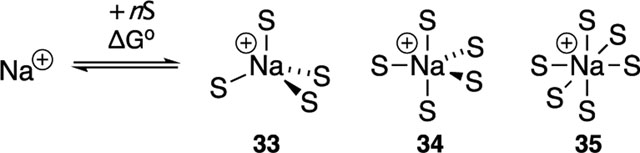
Sodium Cation Solvation.
The solvation energies of sodium cations were calculated (Table 2), filling what appears to be a gap in the computational literature. These computed energies could guide synthetic chemists hoping to markedly enhance reactivity via ionization and to the sodium battery community.74,76 The relative energies corroborate the experimentally determined solvent hierarchy. For example, confirmed ion-pair-forming ligands such as cryptand 32 and HMPA show greater solvation energies than THF, which does not ionize NaHMDS. Furthermore, the solvation energy of the PMDTA-mixed solvates 37 40 demonstrates plausibility for cooperative solvation.
Table 2.
Solvation Energies of Sodium Cations
| ligand | cation | G° (kcal/mol) |
|---|---|---|
| THF | 33m | 66.5 |
| 34m | 71.7 | |
| 35m | 78.5 | |
| HMPA | 33n | 100.2 |
| DMPU | 33o | 92.8 |
| DMF | 34p | 103.3 |
| DMSO | 34q | 97.5 |
| DME | 35v | 80.0 |
| PMDTA | 35w | 77.4 |
| diglyme | 35x | 82.2 |
| 12-crown-4 | +Na(crown)2 | 88.2 |
| 15-crown-S | +Na(crown)2 | 91.5 |
| 18-crown-6 | +Na(crown)2 | 76.6 |
| TDA-1 | +Na(TDA) | 90.0 |
| C222 | +Na(crypt) | 103.7 |
| NIPA | 36 | 115.7 |
| PMDTA + nHMPA | 37 | 81.4 |
| 38 | 96.4 | |
| PMDTA + nDMPU | 39 | 80.0 |
| 40 | 92.4 |
 |
(11) |
CONCLUSION
Exploration of NaHMDS dissolved in >30 solvents showed a dominance of disolvated dimers in neat, weakly coordinating solvents and at low concentrations of strongly coordinating monodentate solvents. Intermediate and strongly coordinating solvents as well as a bevy of di-, tri-, and polyfunctional solvents (usually called ligands) elicited deaggregation without exception. Experimental evidence in conjunction with extensive DFT computations implicated monomers with four- and five-coordinate sodium to be the norm. Ionizations in dipolar and polydentate ligands in the form of both triple ions and ion pairs offer interesting views of sodium cation solvation. On several occasions open dimers were detected, although the evidence was not unassailable.
Most solvents behaved as one might expect when placed in the context of LDA, LiHMDS, and NaDA, but not always. TMCDA and sparteine are relatively good ligands for LiHMDS and other organolithiums but show low affinities for NaHMDS. We are reminded of the ultimate truism: sodium and lithium are different metals. Three crown ethers 12-crown-4, 15-crown-5, and 18-crown-6 showed high affinities for NaHMDS but defy consensus by displaying nearly identical binding constants. We suspect few would have predicted this. Probes of relative affinities using binary mixtures uncovered several examples of cooperative solvation, offering creative opportunities to control structure while reminding us that solvent mixtures bring complexities that must be respected. Moreover, substrate complexation during a reaction is merely a variant of cooperative solvation.
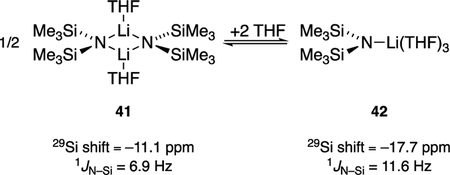
Often new tactical advances emerge from a study that expand our toolkit. The standout example in this study was showing that 15N 29Si coupling first studied by Lukevics and co-workers correlates strongly with the NaHMDS aggregation state. 29Si NMR spectroscopy offered a stupendously convenient window into structure in a variety of solvents over a range of temperatures. We must confess that during studies of [6Li,15N]LiHMDS we never recorded 29Si NMR spectra.14 (We did not need them.) Belatedly, we find that correlations of 15N 29Si hold up, albeit with some quantitative differences when compared to NaHMDS (see 41 and 42). We imagine broader applications to M N(SiR3)2. Moreover, the ease of recording high-quality 29Si NMR spectra suggests tremendous promise akin to tagging a reagent with 19F.77 Casual survey of the literature suggests that, despite the prevalence of silyl groups and silyl-based protecting groups, 29Si NMR spectroscopy is being underutilized.78 It would be a superb tool to monitor reactions and products by the organic synthesis community. We also optimized the basis set, functionals, and grid size for DFT computations of sodium salts to improve our previous protocols. The computations proved invaluable, but the correlations of theory and experiment are qualitative.
What is gained by knowing detailed structures of NaHMDS beyond merely plugging a glaring hole in the organosodium literature? The empirically minded consumers now have a large choice of solvents and can, at least in principle, pursue changes in reactivity and selectivity by targeting observable changes in the underlying aggregation and solvation states. Of course, the mechanism is more complex than that, but it is a start. We hasten to add that it would be difficult to predict something as simple as the solvent-dependent relative reactivities of NaHMDS. Do they span an order of magnitude or 6 orders of magnitude? We also have a particular fondness for affiliating solvent-dependent rates and selectivities with explicit changes in the mechanisms. The assigned solvation and aggregation states of NaHMDS are non-negotiable prerequisites to ongoing detailed mechanistic studies.
The potential synthetic importance of several of the solvents is worthy of comment. MTBE cleanly affords exclusively disolvated dimer and seems like it may be the cosolvent of choice when additional ligands are added. Similarly, some stellar properties of LiHMDS/Et3N/toluene15 in conjunction with foreshadowing from NaHMDS/Et3N-mediated enolizations of acylated oxazolidinones to generate Evans enolates15 suggest NaHMDS/Et3N/toluene could offer superior reactivities at low cost. Ongoing studies of NaDA suggest that PMDTA may play a central role in organosodium chemistry. Lastly, although NaTMDS only supported MCV studies, we wonder if NaTMDS may have undiscovered reactivities, especially for C N bond-forming nucleophilic reactions demanding a smaller nucleophile.
EXPERIMENTAL SECTION
Reagents and Solvents.
Hydrocarbons, monofunctional trialkylamines, monofunctional ethers, HMPA, TMEDA, (R,R)-TMCDA, ( )-sparteine, PMDTA, and diglyme were distilled from blue or purple solutions containing sodium benzophenone ketyl. Styrene, DMPU, DMF, DMSO, 12-crown-4, 15-crown-5, and TDA-1 were dried over 4 Å mol sieves prior to use. Bipyridine and [2.2.2]-cryptand were purchased and used without purification. 18-crown-6 was distilled.
NMR Spectroscopic Analyses.
An NMR tube under vacuum was flame-dried on a Schlenk line, allowed to cool to room temperature, backfilled with argon, placed in a 78 °C dry ice/acetone bath, and charged with NaHMDS and solvents using stock solutions. The sample was mixed with a vortex mixer. Standard 1H, 13C, 15N, and 29Si spectra were recorded on a 500 MHz spectrometer at 500, 125.79, 50.66, and 99.36 MHz, respectively. The chemical shifts are referenced at 120 °C as follows: 1H (Me4Si, 0.0 ppm), 13C (Me4Si, 0.0 ppm), 15N (neat Me2NEt, 25.7 ppm), and 29Si (Me4Si, 0.0 ppm).
[15N]Hexamethyldisilazane.
[15N]NH3 was generated by a known procedure12 by mixing [15N] ammonium chloride (3.0 g, 55.0 mmol, >99 15N isotopic purity) with 6.00 g (150 mmol) of granular NaOH in a 25 mL one-neck round-bottom flask equipped with an NaOH-filled tube through which the ammonia gas is transferred to an empty 100 mL round-bottom flask cooled to 78 °C (see Supporting Information). The mixture was warmed with a heat gun for approximately 20 min. After the transfer of ammonia was complete, l-(trimethylsilyl)imidazole (14.7 g, 15.3 mL, 105 mmol, 98 purity) was added at 78 °C with stirring. Imidazole precipitated immediately, after which anhydrous diethyl ether (20 mL) was added to the flask, and the mixture was held at 0 °C for 40 min. Cholesterol (3.0 g) was added to the [15N]hexamethyldisilazane with stirring for 45 min to remove excess l-(trimethylsilyl)imidazole. Short path distillation at atmospheric pressure removed the diethyl ether. Vacuum distillation (40 mmHg, 20 °C) a orded 4.75 mL (47 yield) of (Me3Si)215NH.
[15N]-Sodium Hexamethyldisilazide 1).
[15N]NaHMDS was prepared following a known procedure.12 To a flame-dried fine-mesh swivel-frit setup was added sliced sodium metal (1.20 g, 52.4 mmol) in a glovebox. The apparatus was moved to a Schlenk line for the remainder of the procedure. Under argon, [15N]HMDS (7.31 g, 9.45 mL, 45.0 mmol) and 40 mL of DMEA were added to the reaction flask at room temperature. Isoprene (2.62 mL, 26.2 mmol) dissolved in 8 mL of dry DMEA was then added over 1 h via syringe pump to the mixture. After addition of isoprene, the reaction was stirred at RT for an additional 2 h. The solution was subsequently filtered through a frit, transferred with a canula to a second swivel coarse-frit setup, and vacuum evaporated to dryness for at least 10 h to yield a white powder. The powder was suspended in dry pentane (~20 mL), stirred for 1 h, and filtered. Finally, the product was washed with 20 mL of pentane and yielded 6.70 g (91 yield) of [15N]NaHMDS as a white solid,12 which was transferred to a glovebox and stored at room temperature. NaHMDS can be recrystallized as previously described12 but with no detectable improvement. 1H NMR (toluene-d8, 500 MHz): δ 0.2 (s, 18 H). 13C{1H} NMR (toluene-d8, 125.72 MHz): δ 6.8 (d, 2JN C = 2.7 Hz). 29Si NMR (toluene-d8, 99.36 MHz): δ 14.4 (d, 1JN Si = 7.9 Hz).
Sodium Tetramethyldisilazide NaTMDS, 17).
NaTMDS was synthesized from 1,1,3,3-tetramethyldisilazane using the prep described for NaHMDS, a ording NaTMDS (17) as a white solid (3.0 g, 50 yield).17 13C{1H} NMR (toluene-d8, 125.72 MHz): δ 5.9. 29Si NMR (toluene-d8, 99.36 MHz): δ 25.7.
Sodium Bis dimethyl phenyl)silyl)amide NaDPTMDS, 18).
NaDPTMDS was synthesized from bis (dimethyl(phenyl)silyl) amine using the same prep described for NaHMDS, a ording NaDPTMDS (18) as a white solid (5.3 g, 45 yield).18 13C{1H} NMR (125.72 MHz, toluene-d8): δ 148.6, 133.2, 5.5.
Sodium 1-Aza-2,2,5,5-tetramethyl-2,5-disilacyclopentane 19).
Sodiated l-aza-2,2,5,5-tetramethyl-2,5-disilacyclopentane was prepared using the same dissolving-metal-based prep described previously for NaHMDS, a ording 19 as a white solid (3.4 g, 70; yield).19 13C{1H} NMR (125.72 MHz, DMEA): δ 13.2, 6.3.
Crystallization Conditions 13z and 14cc).
To a flame-dried NMR tube under Ar was added 600 μL of 0.10 M NaHMDS in DMEA at room temperature. Next, 1.0 equiv of ligand was added to the tube, which immediately resulted in a crystallization event. The tube was then sealed under partial vacuum. Recrystallization of each, complex was conducted by simply running crystals suspended in DMEA under hot tap water followed by slow cooling until they reached room temperature.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (GM131713) for support and Samantha N. MacMillan for the crystal structures.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c02546.
Spectroscopic data, rate, and computational data (PDF)
Accession Codes
CCDC 2042630 contains the supplementary crystallographic, data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.joc.0c02546
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Seyferth D Alkyl and Aryl Derivatives of the Alkali Metals: Useful Synthetic Reagents as Strong Bases and Potent Nucleophiles. 1. Conversion of Organic Halides to Organoalkali-Metal Compounds. Organometallics 2006, 25, 2. [Google Scholar]; (b) Seyferth D Alkyl and Aryl Derivatives of the Alkali Metals: Strong Bases and Reactive Nucleophiles. 2. Wilhelm Schlenks Organoalkali-Metal Chemistry. The Metal Displacement and the Transmetalation Reactions. Metalation of Weakly Acidic Hydrocarbons. Superbases. Organometallics 2009, 28, 2. [Google Scholar]; (c) Lochmann L; Janata M 50 Years of Superbases Made from Organolithium Compounds and Heavier Alkali Metal Alkoxides. Eur. J. Chem 2014, 12, 537. [Google Scholar]; (d) Robertson SD; Uzelac M; Mulvey RE Alkali-Metal-Mediated Synergistic Effects in Polar Main Group Organometallic Chemistry. Chem. Rev 2019, 119, 8332. [DOI] [PubMed] [Google Scholar]
- (2).Woltornist RA; Ma Y; Algera RF; Zhou Y; Zhang Z; Collum DB Structure, Reactivity, and Synthetic Applications of Sodium Diisopropylamide. Synthesis 2020, 52, 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Algera RF; Ma Y; Collum DB Sodium Diisopropylamide: Aggregation, Solvation, and Stability. J. Am. Chem. Soc 2017, 139, 7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Sodium hexamethyldisilazide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Watson BT; Lebel H, Eds.; John Wiley & Sons: New York, 2005; pp 1 10. [Google Scholar]
- (5).(a) Stumpf A; Cheng ZK; Wong B; Reynolds M; Angelaud R; Girotti J; Deese A; Gu C; Gazzard L Development of an Expedient Process for the Multi-Kilogram Synthesis of Chkl Inhibitor GDC-0425. Org. Process Res. Dev 2015, 19, 661. [Google Scholar]; (b) Kerdesky FAJ; Leanna MR; Zhang J; Li W; Lallaman JE; Ji J; Morton HE An Efficient Multikilogram Synthesis of ABT-963: A Selective COX-2 Inhibitor. Org. Process Res. Dev 2006, 10, 512. [Google Scholar]; (c) Butters M; Ebbs J; Green SP; MacRae J; Morland MC; Murtiashaw CW; Pettman AJ Process Development of Voriconazole: A Novel Broad-Spectrum Triazole Antifungal Agent. Org. Process Res. Dev 2001, 5, 28. [Google Scholar]; (d) Fuerstner A; Fenster MDB; Fasching B; Godbout CP; Radkowski K Toward the Total Synthesis of Spirastrellolide A. Part 2: Conquest of the Northern Hemisphere. Angew. Chem. Int. Ed 2006, 45, 5510. [DOI] [PubMed] [Google Scholar]; (e) Davis FA Recent Applications of N-Sulfonyloxaziridines (Davis oxaziridines) in Organic Synthesis. Tetrahedron 2018, 74, 3198. [Google Scholar]; (f) Rao KS; St-Jean F; Kumar A Quantitation of a Ketone Enolization and a Vinyl Sulfonate Stereoisomer Formation Using Inline IR Spectroscopy and Modeling. Org. Process Res. Dev 2019, 23, 945. [Google Scholar]
- (6).(a) 1: Driess M; Pritzkow H; Skipinski M; Winkler U Synthesis and Solid State Structures of Sterically Congested Sodium and Cesium Silyl(fluorosilyl)phosphanide Aggregates and Structural Characterization of the Trimeric Sodium Bis (trimethylsilyl) amide. Organometallics 1997, 16, 5108. [Google Scholar]; (b) 2: Kennedy AR; Mulvey RE; O Hara CT; Robertson SD; Robertson GM Catena-Poly [Sodium-μ2-(N N N N - Tetramethylethane-1,2-Diamine)-y2-N N -Sodium- Bis [μ2-Bis (Trimethylsilyl) Azanido-2 N:N]]. Acta Crystallogr. Sect. E: Struct. Rep. Online 2012, 68, No. ml468. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) 3, 10, and 11: Schuler P; Gorls H; Westerhausen M; Krieck S Bis (trimethylsilyl) amide Complexes of s-Block Metals with Bidentate Ether and Amine Ligands. Dalton Trans 2019, 48, 8966. [DOI] [PubMed] [Google Scholar]; (d) 4 and 5: Ojeda-Amador AI; Martínez-Martínez AJ; Kennedy AR; Armstrong DR; O Hara CT Monodentate Coordination of the Normally Chelating Chiral Diamine (R R) -TMCDA. Chem. Commun 2017, 53, 324. [DOI] [PubMed] [Google Scholar]; (e) 6: Sarazin Y; Coles SJ; Hughes DL; Hursthouse MB; Bochmann M Cationic Brønsted Acids for the Preparation of Sn(lV) Salts: Synthesis and Characterisation of [Ph3Sn(OEt2)] [H2N{B(C6F5)3}2],[Sn(NMe2)3(HNMe2)2] [B-(C6F5)4] and [Me3Sn(HNMe2)2][B(C6F5)4]. Eur. J. Inorg. Chem 2006, 2006, 3211. [Google Scholar]; (f) 7: Karl M; Seybert G; Massa W; Harms K; Agarwal S; Maleika R; Stelter W; Greiner A; Neumüller WH; Dehnicke K Amidometallate von Seltenerdelementen. Synthese Und Kristallstrukturen von [Na(12-Krone-4)2][M{N-(SiMe3)2}3(OSiMe3)] (M = Sm, Yb), [Na(THF)3Sm{N-(SiMe3)2}3(C=C-Ph)], [Na(THF)6][Lu2(μ-NH2)(μ-NSiMe3){N-(SiMe3)2}4] Sowie von [NaN(SiMe3)2(THF)]2. Z. Anorg. Allg. Chem 1999, 625, 1301. [Google Scholar]; (g) 8: Neufeld R; Michel R; Herbst-Irmer R; Schöne R; Stalke D Introducing a Hydrogen-Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexame- thyldisilazides (MHMDS, M = Li, Na, K, Rb, and Cs) with Ammonia. Chem. - Eur. J 2016, 22, 12340. [DOI] [PubMed] [Google Scholar]; (h) 9: Edelmann FT; Pauer F; Wedler M; Stalke D Preparation and Structural Characterization of Dioxane Coordinated Alkali Metal Bis (Trimethylsilyl) Amides. Inorg. Chem 1992, 31, 4143. [Google Scholar]; (i) For a NaHMDS sodium enolate mixed aggregate characterized crystallographically, see:; Williard PG; Hintze MJ Mixed Aggregates: Crystal Structures of a Lithium Ketone Enolate/Lithium Amide and of a Sodium Ester Enolate/Sodium Amide. J. Am. Chem. Soc 1990, 112, 8602. [Google Scholar]
- (7).(a) Knapp C; Lork E; Borrmann T; Stohrer W-D; Mews R Versuche Zur Darstellung Vont-BuCN5S3 Und Die Unerwartete Isolierung Einer Kovalenten Modifikation von Tetraschwefelpentas- tickstoff-Chlorid S4N5Cl. Z. Anorg. Allg. Chem 2005, 631, 1885. [Google Scholar]; (b) Clark NM; Garcia-Àlvarez P; Kennedy AR; O Hara CT; Robertson GM Reactions of ( )-Sparteine with Alkali Metal HMDS Complexes: Conventional Meets the Unconventional. Chem. Commuti 2009, 39, 5835. [DOI] [PubMed] [Google Scholar]; (c) Williard PG; Nichols MA Structural Characterization of Mixed Alkali Metal Bis (trimethylsilyl) Amide Bases. J. Am. Chem. Soc 1991, 113, 9671. [Google Scholar]
- (8).Ojeda-Amador AI; Martinez-Martinez AJ; Kennedy AR; O Hara CT Synthetic and Structural Studies of Mixed Sodium Bis (trimethylsilyl) Amide/Sodium Halide Aggregates in the Presence of η2 -N N-, η3 -N N N/N O N-, and η4 -N N N N-donor Ligands. Inorg. Chem 2015, 54, 9833. [DOI] [PubMed] [Google Scholar]
- (9).Kupce E; Lukevics E; Varezhkin YM; Mikhailova AN; Sheludyakov VD Silicon-29-Nitrogen-15 Spin-Spin Coupling Constants in Silazanes. Organometallics 1988, 7, 1649. [Google Scholar]; and references cited therein.
- (10).For an extensive review on the chemistry of the alkali metal amides, see:; Mulvey RE; Robertson SD Synthetically Important Alkali-Metal Utility Amides: Lithium, Sodium, and Potassium Hexamethyldisilazides, Diisopropylamides, and Tetramethylpiperidides. Angew. Chem. Int. Ed 2013, 52, 11470. [DOI] [PubMed] [Google Scholar]
- (11).Luo G; Luo Y; Qu J Direct Nucleophilic Trifluoromethylation Using Fluoroform: a Theoretical Mechanistic Investigation and Insight into the Effect of Alkali Metal Cations. New J. Chem 2013, 37, 3274. [Google Scholar]
- (12).Woltornist RA; Collum DB Using 15N-29Si Scalar Coupling to Determine Aggregation and Solvation States. J. Am. Chem. Soc 2020, 142, 6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13). Many of the results for LiHMDS and LDA can be accessed through two review articles.14,16 More recent studies not cited therein are provided below.15.
- (14).Lucht BL; Collum DB Lithium Hexamethyldisilazide: A View of Lithium Ion Solvation Through a Glass-Bottom Boat. Acc. Chem. Res 1999, 32, 1035. [Google Scholar]
- (15).Snaddon TN; Buchgraber P; Schulthoff S; Wirtz C; Mynott R; Fürstner A Total Synthesis of Berkelic Acid. Chem. - Eur. J 2010, 16, 12133. [DOI] [PubMed] [Google Scholar]
- (16).Collum DB Solution Structures of Lithium Dialkylamides and Related N-Lithiated Species: Results from 6Li-15N Double; Labeling Experiments. Acc. Chem. Res 1993, 26, 227. [Google Scholar]
- (17).(a) Eppinger J; Herdtweck E; Anwander R Synthesis and Characterisation of Alkali Metal Bis(Dimethylsilyl) Amides: Infinite All-Planar Laddering in the Unsolvated Sodium Derivative. Polyhedron, 1998, 17, 1195. [Google Scholar]; (b) Schneider J; Popowski E; Reinke H Darstellung, Charakterisierung Und Reaktionsverhalten von Natrium-Und Kaliumhydridosilylamiden R2(H)Si-N(M)R (M = Na, K) - Kristallstruktur von [(ME3C)2(H)Si-N(K)SiMe3]2 ·THF. Z Anorg. Allg. Chem 2003, 629, 55. [Google Scholar]
- (18).Evans WJ; Rego DB; Ziller JW Lanthanum and Alkali; Metal Coordination Chemistry of the Bis (Dimethylphenylsilyl)Amide Ligand. Inorg. Chem 2006, 45, 3437. [DOI] [PubMed] [Google Scholar]
- (19).1-Aza-2,2,5,5-tetramethyl-2,5-disilacyclopentane preparation:; Baney RH; Haberland GG The Question of Flexibility Around the Silicon-Nitrogen Bond in Polysilazanes. A Comparison with Polysiloxanes. J. Organomet. Chem 1966, 5, 320 325. [Google Scholar]
- (20).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Zakrzewski VG; Montgomery JA Jr.; Stratmann RE; Burant JC; Dapprich S; Millam JM; Daniels AD; Kudin KN; Strain MC; Farkas O; Tornasi J; Barone V; Cossi M; Cammi R; Mennucci B; Pomelli C; Adamo C; Cli ord S; Ochterski J; Petersson GA; Ayala PY; Cui Q; i Morokuma K; Malick DK; Rabuck AD; Raghavachari K; Foresman JB; Cioslowski J; Ortiz JV; Baboul AG; Stefanov BB; Liu G; Liashenko A; Piskorz P; Komaromi I; Gomperts R; Martin RL; Fox DJ; Keith T; Al-Laham MA; Peng CY; Gill A; Nanayakkara C; Gonzalez M; Challacombe PMW; Johnson i B.; Chen W; Wong MW; Andres JL; Gonzalez C; Head-Gordon M; Replogle ES; Pople JA Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- (21).Zhao Y; Truhlar DG The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc 2008, 120, 215. [Google Scholar]
- (22). Several seemingly simple computations at the MP2 level of theory failed for reasons that were unclear. This is not a problem using DFT with M06 functionals.
- (23).Weigend F; Ahlrichs R Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys 2005, 7, 3297. [DOI] [PubMed] [Google Scholar]
- (24).Legault CY CYLview 1.0b; Université de Sherbrooke, 2009. (http://www.cylview.org). [Google Scholar]
- (25).Bootsma AN; Wheeler S Popular Integration Grids Can Result in Large Errors in DFT-Computed Free Energies. ChemRxiv 2019. [Google Scholar]
- (26). For example, the solvation energies of toluene on NaHMDS dimer decreased by 2.0 kcal/mol by switching from a grid size of 75 302 to 99 590.
- (27).Yang Z; Yang S; Yu P; Li Y; Doubleday C; Park J; Patel A; Jeon B.-s.; Russell WK; Liu H.-w.; Russell DH; Houk KN Influence ofwater and enzyme SpnF on the dynamics and energetics of the ambimodal [6 + 4]/[4 + 2] cycloaddition. Proc. Natl. Acad. Sei. U. S. A 2018, 115, E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Renny JS; Tomasevich LL; Tallmadge EH; Collum DB Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed 2013, 52, 11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).The intended mole fraction refers to the mole fraction based on what was added to the samples. The measured mole fraction the mole fraction within only the ensemble of interest eliminates the distorting effects of impurities. This problem has been highlighted:; Brynn Hibbert D; Thordarson P The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun 2016, 52, 12792. [DOI] [PubMed] [Google Scholar]
- (30).Job P Formation and Stability of Inorganic Complexes in Solution. Ann. Chim 1928, 9, 113. [Google Scholar]
- (31). The concentration of NaHMDS, although expressed in units of molarity, refers to the concentration of the monomer subunit (normality).
- (32).Yamada S Cation- Interactions in Organic Synthesis. Chem. Rev 2018, 118, 11353. [DOI] [PubMed] [Google Scholar]
- (33).Neufeld R; John M; Stalke D The Donor-Base-Free Aggregation of Lithium Diisopropyl Amide in Hydrocarbons Revealed by a DOSY Method. Angew. Chem. Int. Ed 2015, 54, 6994. [DOI] [PubMed] [Google Scholar]
- (34).Lambert C; von Ragué Schleyer P Are Polar Organometallic Compounds “Carbanions”? The Gegenion Effect on Structure and Energies of Alkali-Metal Compounds. Angew. Chem. Int. Ed. Engl 1994, 33, 1129. [Google Scholar]
- (35).(a) Eastham JF; Gibson GW Solvent Effects in Organometallic Reactions. II. J. Am. Chem. Soc 1963, 85, 2171. [Google Scholar]; (b) Bartlett PD; Goebel CV; Weber WP Ethylenation of Secondary and Tertiary Alkyllithiums. IL Its Kinetics and the Nature of the Active Species. J. Am. Chem. Soc 1969, 91, 7425. [Google Scholar]; (c) Lewis HL; Brown TL Association of Alkyllithium Compounds in Hydrocarbon Media. Alkyllithium-Base Interactions. J. Am. Chem. Soc 1970, 92, 4664. [Google Scholar]; (d) Popov AI Alkali Metal NMR and Vibrational Spectroscopic Studies on Solvates in Non-aqueous Solvents. Pure Appl. Chem 1975, 41, 275. [Google Scholar]
- (36). Double solvation of dimer follows a first- rather than second- order saturation function because of a single solvent per sodium.
- (37).(a) Reich HJ Role of Organolithium Aggregates and Mixed Aggregates in Organolithium Mechanisms. Chem. Rev 2013, 113, 7130. [DOI] [PubMed] [Google Scholar]; (b) Hilmersson G; Davidsson O A Multinuclear NMR-Study of a Chiral Lithium Amide with an Intramolecular Chelating Methoxy Group in Coordinating Solvents at the Slow Ligand-Exchange Limit. J. Org. Chem 1995, 60, 7660. [Google Scholar]; (c) Hilmersson G; Ahlberg P; Davidsson O Enantiomeric Perturbation of Equilibria. Differential Solvation of a Chiral Lithium Amide by the Enantiomers of 2- Methyltetrahydrofuran Measured by NMR Spectroscopy. J. Am. Chem. Soc 1996, 118, 3539. [Google Scholar]
- (38). We use the term “cooperative” for advantageous (protagonistic) influences of two coordinated solvents. The term “correlated” is intended as a neutral term in which two or more coordinated solvents influence their relative binding irrespective of whether protagonistically or antagonistically.40 Correlated ligation is an issue across all metals.41 There is also a statistical factor in a serial; substitution that is easily overlooked.42.
- (39).Hoffmann D; Collum DB Competitive and Cooperative Binding of Vicinal Diamines to n-Butyllithium Dimers: Relationship of Ligand Structure and Relative Solvation Energies. J. Am. Chem. Soc 1998, 120, 5810. [Google Scholar]
- (40).For investigations of correlated phosphine binding on transition metals, see:; Li C; Oliván M; Nolan S; Caulton KG Organometallics 1997, 16, 4223. [Google Scholar]; and references cited therein.
- (41).(a) Benson SW Statistical Factors in the Correlation of Rate Constants and Equilibrium Constants. J. Am. Chem. Soc 1958, 80, 5151. [Google Scholar]; (b) Fay RC; Lowry RN Proton Magnetic Resonance Studies of Ligand-Exchange Equilibriums for Dihalo- and Diethoxybis(beta-diketonato)titanium(IV) Complexes. Inorg. Chem 1974, 13, 1309. [Google Scholar]
- (42).The computations use the Gaussian standard state of 1.0 atm. If the solvent concentration is corrected to neat solvent (approximately 10 M), each solvation step benefits from approximately 2.0 kcal/mol of additional stabilization at 78 °C (195 K).; Pratt LM; Merry S; Nguyen SC; Quan P; Thanh BT A Computational Study of Halomethyllithium Carbenoid Mixed Aggregates with Lithium Halides and Lithium Methoxide. Tetrahedron 2006, 62, 10821. [Google Scholar]
- (43). From Wikipedia, “an isodesmic reaction is a chemical reaction in which the type of chemical bonds broken in the reactant are the same as the type of bonds formed in the reaction product”.
- (44).Ojeda-Amador AI; Martinez-Martinez AJ; Robertson GM; Robertson SD; Kennedy AR; O’Hara CT Exploring the Solid State and Solution Structural Chemistry of the Utility Amide Potassium Hexamethyldisilazide (KHMDS). Dalton Trans 2017, 46, 6392. [DOI] [PubMed] [Google Scholar]
- (45).(a) Klinkhammer KW; Klett J; Xiong Y; Yao S Homo- and Heteroleptic Hypersilylcuprates Valuable Reagents for the Synthesis of Molecular Compounds with a Cu-Si Bond. Eur. J. Inorg. Chem 2003, 2003, 3417. [Google Scholar]; (b) Schumann H; Hummert M; Lukoyanov AN; Fedushkin IL Sodium Cation Migration Above the Diimine pi-System of Solvent Coordinated dpp-BIAN Sodium Aluminum Complexes (dpp-BIAN = 1,2-Bis[(2,6-diisopropylphenyl)imino]acenaphthene). Chem. Eur. J 2007, 13, 4216. [DOI] [PubMed] [Google Scholar]
- (46).Morita H; Van Beylen M New Vistas on the Anionic Polymerization of Styrene in Non-Polar Solvents by Means of Density Functional Theory. Polymers 2016, 8, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Gutmann V The Donor-Acceptor Approach to Molecular Interactions; Plenum: New York, 1978. [Google Scholar]
- (48).(a) Seligson AL; Trogler WC Cone Angles for Amine Ligands. X-ray Crystal Structures and Equilibrium Measurements for Ammonia, Ethylamine, Diethylamine, and Triethylamine Complexes with the [bis(Dimethylphosphino)ethane]methylpalladium(II) Cation. J. Am. Chem. Soc 1991, 113, 2520. [Google Scholar]; (b) Choi M-G; Brown TL A Molecular Mechanics Model of Ligand Effects. 4. Binding of Amines to Chromium Pentacarbonyl: ER Values for Amines. Inorg. Chem 1993, 32, 1548. [Google Scholar]; (c) Widenhoefer RA; Buchwald SL Formation of Palladium Bis (amine) Complexes from Reaction of 1 Amine with Palladium Tris(o-tolyl)phosphine Mono(amine) Complexes. Organometallics 1996, 15, 3534. [Google Scholar]
- (49).Zhang Z; Collum DB Structures and Reactivities of Sodiated Evans Enolates: Role of Solvation and Mixed Aggregation on the Stereochemistry and Mechanism of Alkylations. J. Am. Chem. Soc 2019, 141, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).(a) Jarek RL; Denson SC; Shin SK Solvation of the Li+- Cl -Li+ Triple Ion in the Gas Phase. J. Chem. Phys 1998, 109, 4258. [Google Scholar]; (b) Chapman DM; Hester RE Ab Initio Conformational Analysis of 1,4-Dioxane. J. Phys. Chem. A 1997, 101, 3382. [Google Scholar]
- (51).(a) Kaga A; Hayashi H; Hakamata H; Oi M; Uchiyama M; Takita R; Chiba S Nucleophilic Amination of Methoxy Arenes Promoted by a Sodium Hydride/Iodide Composite. Angew. Chem. Int. Ed 2017, 56, 11807. [DOI] [PubMed] [Google Scholar]; (b) Pang JH; Ong DY; Watanabe K; Takita R; Chiba S Leaving Group Ability in Nucleophilic Aromatic Amination by Sodium Hydride-Lithium Iodide Composite. Synthesis 2020, 52, 393. [Google Scholar]
- (52).(a) Pang JH; Kaga A; Roediger S; Lin MT; Chiba S Revisiting the Chichibabin Reaction: C2 Amination of Pyridines with a NaH-Iodide Composite. Asian J. Org. Chem 2019, 8, 1058. [Google Scholar]; (b) Ezquerra J; Alvarez-Builla J Org. Sonochem. A Facile Synthesis of 1-Methylisoquinoline. Org. Prep. Proced. Int 1985, 17, 190. [Google Scholar]; (c) Pang JH; Kaga A; Chiba S Nucleophilic Amination of Methoxypyridines by a Sodium Hydride-Iodide Composite. Chem. Commun 2018, 54, 10324. [DOI] [PubMed] [Google Scholar]; (d) Narayan S; Seelhammer T; Gawley RE Microwave-Assisted Solvent-Free Amination of Halo-(Pyridine or Pyrimidine) without Transition Metal Catalyst. Tetrahedron Lett 2004, 45, 757. [Google Scholar]; (e) Garia A; Jain N Transition-Metal-Free Synthesis of Fused Quinazolinones by Oxidative Cyclization of N-Pyridylindoles. J. Org. Chem 2019, 84, 9661. [DOI] [PubMed] [Google Scholar]
- (53).Oliveri IP; Maccarrone G; Di Bella S A Lewis Basicity Scale in Dichloromethane for Amines and Common Nonprotogenic Solvents Using a Zinc(II) Schiff-Base Complex as Reference Lewis Acid. J. Org. Chem 2011, 76, 8879. [DOI] [PubMed] [Google Scholar]
- (54).Chakrabarti P; Dunitz JD Directional Preferences of Ether O-Atoms Towards Alkali and Alkaline Earth Cations. Helv. Chim. Acta 1982, 65, 1482. [Google Scholar]
- (55).(a) Polyamine-Chelated Alkali Metal Compounds; Langer AW Jr., Ed.; American Chemical Society: Washington, DC, 1974. [Google Scholar]; (b) Collum DB Is N N N N -Tetramethylethylenediamine a Good Ligand for Lithium? Acc. Chem. Res 1992, 25, 448. [Google Scholar]
- (56).Bauer W; Schleyer P. v. R. Advances in Carbanion Chemistry, Snieckus V, ed.; JAI: New York, 1992; p 89. [Google Scholar]
- (57).Pratt LM; Mogali S; Glinton K Solvent Effects on the Aggregation State of Lithium Dialkylaminoborohydrides. J. Org. Chem 2003, 68, 6484. [DOI] [PubMed] [Google Scholar]
- (58).(a) Cabello N; Kizirian JC; Alexakis A Enantioselective Addition of Aryllithium Reagents to Aromatic Imines Mediated by 1,2-Diamine Ligands. Tetrahedron Lett 2004, 45, 4639. [Google Scholar]; (b) Mealy MJ; Luderer MR; Bailey WF; Sommer MB Effect of Ligand Structure on the Asymmetric Cyclization of Achiral Olefinic Organolithiums. J. Org. Chem 2004, 69, 6042. [DOI] [PubMed] [Google Scholar]; (c) Cointeaux L; Alexakis A Enantioselective Addition of Organolithium Reagents to Quinoline Catalyzed by 1,2-Diamines. Tetrahedron: Asymmetry 2005, 16, 925. [Google Scholar]; (d) Kizirian JC; Cabello N; Pinchard L; Caille JC; Alexakis A Enantioselective Addition of Methyllithium to Aromatic Imines Catalyzed by C2 Symmetric Tertiary Diamines. Tetrahedron 2005, 61, 8939. [Google Scholar]
- (59).Van Leeuwen PWNM; Kamer PCJ; Reek JNH; Dierkes P Ligand Bite Angle Effects in Metal-Catalyzed C-C Bond Formation. Chem. Rev 2000, 100, 2741. [DOI] [PubMed] [Google Scholar]
- (60).( )-Sparteine. Hoppe D; Morgan BJ; Kozlowski MC In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp 1 6. [Google Scholar]
- (61).Garcia-Alvarez P; Kennedy AR; O Hara CT; Reilly K; Robertson GM Synthesis and Structural Chemistry of Alkali Metal tris(HMDS) Magnesiates Containing Chiral Diamine Donor Ligands. Dalton Trans 2011, 40, 5332. [DOI] [PubMed] [Google Scholar]
- (62).(a) Tian Z; Fattahi A; Lis L; Kass SR Neutral Intramolecular Hydrogen-Bonded Bases. Croat. Chem. Acta 2009, 82, 41. [Google Scholar]; (b) Maksić ZB; Kovačević B; Vianello R Advances in Determining the Absolute Proton Affinities of Neutral Organic Molecules in the Gas Phase and Their Interpretation: A Theoretical Account. Chem. Rev 2012, 112, 5240. [DOI] [PubMed] [Google Scholar]
- (63).Lannert KP; Joesten MD Metal Complexes of Nonamethylimidodiphosphoramide. Inorg. Chem 1968, 7, 2048. [Google Scholar]; (b) Bokolo K; Delpuech JJ; Rodehüser L; Rubini P; Courtois A; Elkaim E; Protas J; Rinaldi D Conformations of the Nonbonded and the Coordinated Ligand Nonamethylimidodiphosphoramide (NIPA) in the Solid State and in Solution. X-Ray Structure Determinations, NMR Study, and Theoretical Calculations on the NIPA Molecule and the Complex [UO2(NIPA)2C2H. J. Am. Chem. Soc 1984, 106, 6333. [Google Scholar]
- (64).Collum DB; Cho B unpublished.
- (65).(a) Rodehuser L; Chniber T; Rubini P; Delpuech JJ Mixed Ligand Complexes Containing β-diphosphoramides. I. Substitution of DMSO by NIPA in Solvates of Cadmium(II). A Cadmium-113 NMR Study. Inorg. Chim. Acta 1988, 148, 227. [Google Scholar]; (b) Tomoi M; Onozawa T; Seki K; Kakiuchi H Anionic Polymerization of Vinyl Monomers with Organometallic Compound Phosphoramide System. Polym. J 1975, 7, 372. [Google Scholar]
- (66).Rubini PR; Rodenhueser L; Delpuech JJ A Nuclear, Magnetic Resonance Study of Metal Complexes of Nonamethylimidodiphosphoramide. Inorg. Chem 1979, 18, 2962. [Google Scholar]
- (67).Cossentini M; Strzalko T; Seyden-Penne J A Study of the Michael Reaction in the Presence of Complexing Agents for Alkali Metal Cations in Aprotic Media or Under Phase-Transfer Conditions. Bull. Soc. Chim. France 1987, 531. [Google Scholar]
- (68).Delville A; Detellier C; Gerstmans A; Laszlo P Preferential Solvation of the Sodium Cation in Binary Mixtures of Tetrahydrofuran with Polyamines, in Relation with the Chelate Effect. Helv. Chim. Acta 1981, 64, 556. [Google Scholar]
- (69).Fraenkel G; Subramanian S; Chow A The Carbon-Lithium Bond in Monomeric Aryllithiums: Dynamics of Exchange, Relaxation, and Rotation. J. Am. Chem. Soc 1995, 117, 6300. [Google Scholar]
- (70).Crown sodium solvent complexes are prevalent in the crystallographic literature. While there are a couple of THF/15-crown-5 complexes in the Cambridge Database, donor solvent/18-crown-6 complexes are commonplace. No evidence for 12-crown-4 mixed solvates could be found,; (a) Campazzi E; Solari E; Scopelliti R; Floriani C Lanthanide Organometallic Chemistry Based on the Porphyrinogen Skeleton: Acetylene and Ethylene Bridging Praseodymium and Neodymium H5:H1:H5:H1-Bonded to Meso-Octaethyl-porphyrinogen. Chem. Commun 1999, 17, 1617. [Google Scholar]; (b) Uhl W; Hannemann F A Methylene Bridged Dialuminium Compound as a Chelating Lewis Acid - Complexation of Azide and Acetate Anions by R2Al-CH2-AlR2 [R = CH(SiMe3)2]. J. Organomet. Chem 1999, 579, 18. [Google Scholar]; (c) Hervé A; Thuéry P; Ephritikhine M; Berthet JC Structural Diversity in Cyanido Thorocene Complexes. Organometallics 2014, 33, 2088. [Google Scholar]; (d) Cambillau C; Bram G; Corset J; i Riche C Complexes Formés Par Addition d éthers-Couronnes Aux Enolates de Na+ et K+ de 1 Acétylacétate d éthyle: Structures, Cristallines et Système d équilibres En Solution Dans le THF et le DMSO. Can. J. Chem 1982, 60, 2554. [Google Scholar]
- (71). Titrating a solution of NaHMDS in toluene or MTBE with two ligands (two crowns, for example) will necessarily produce solvates of both at up to 0.5 equiv of each. Only when an excess has been added ‘ can the preference be established. The protocol is expedient, and the low concentrations serve as control experiments.
- (72).(a) Soula G Tris (polyoxaalkyl) amines (Trident), a New Class of Solid-Liquid Phase-Transfer Catalysts. J. Org. Chem 1985, 50, 3717. [Google Scholar]; (b) Starks CM; Liotta CL; Halpern M Phase-Transfer, Catalysis; Chapman & Hall: New York, 1994. [Google Scholar]
- (73).Xu M; Jupp AR; Qu ZW; Stephan DW Angew. Chem. Int. Ed 2018, 57, 11050. [DOI] [PubMed] [Google Scholar]
- (74).Wang Y; Song S; Xu C; Hu N; Molenda J; Lu L Development of Solid-State Electrolytes for Sodium-Ion Battery-A Short Review. NMS 2019, 1, 91. [Google Scholar]
- (75).There are an enormous number of documented +Na(THF)6 and +Na(κ2-DME)3 gegenions. For an example of both, see:; Livingstone Z; Hernan-Gomez A; Baillie SE; Armstrong DR; Carrella LM; Clegg W; Harrington RW; Kennedy AR; Rentschler E; Hevia E J. Chem. Soc. Dalton Trans 2016, 45, 6175. [DOI] [PubMed] [Google Scholar]
- (76).For a particularly incisive comparison of methods for calculating alkali metal solvation using both explicit and continuum 1 models of solvation (including lithium and sodium solvated by THF and DME), see:; Ziegler MJ; Madura JD Solvation of Metal Cations in Non-aqueous Liquids. J. Solution Chem 2011, 40, 1383. [Google Scholar]
- (77).Review of 19F NMR spectroscopy in organometallic chemistry:; Espinet P; Albeniz AC; Casares JA; Martinez-Ilarduya JM 19F NMR in Organometallic Chemistry: Applications of Fluorinated Aryls. Coord. Chem. Rev 2008, 252, 2180. [Google Scholar]
- (78).Excluding the explicitly silicon-centric world of organosilicon chemistry, one finds remarkably few instances in which 29Si NMR spectroscopy is used by the general organic chemistry community, and most are mechanistic studies. Applications in synthesis include:; (a) Wu N.j Wahl B.j Woodward S; Lewis W 1,4-Addition of TMSCC13 to Nitroalkenes: Efficient Reaction Conditions and Mechanistic Understanding. Chem. - Eur. J 2014, 20, 7718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Humbrias-Martin J; Perez-Aguilar MC; Mas-Balleste R; Dentoni Litta A; Lattanzi A; Della Sala G; Fernandez-Salas JA; Aleman J Enantioselective Conjugate Azidation of α,β-Unsaturated Ketones Under Bifunctional Organocatalysis by Direct Activation of TMSN3. Adv. Synth. Catal 2019, 361, 4790. [Google Scholar]; (c) Kolomeitsev A; Bissky G; Lork E; Movchun V; Rusanov E; Kirsch P; Röschenthaler G-V Different Fluoride Anion Sources and (Trifluoromethyl)trimethylsilane: Molecular Structure of Tris-(dimethylamino)sulfonium Bis(trifluoromethyl)trimethylsiliconate, the First Isolated Pentacoordinate Silicon Species with Five Si-C bonds. Chem. Commun 1999, 1017. [Google Scholar]; (d) Maggiarosa N; Tyrra W; Naumann D; Kirij NV; Yagupolskii YL [Me3Si(CF3)F] and [Me3Si(CF3)2] : Reactive Intermediates in Fluoride-Initiated Trifluoromethylation with Me3SiCF3 An NMR Study. Angew. Chem. Int. Ed 1999, 38, 2252. [DOI] [PubMed] [Google Scholar]; (e) Kobayashi S; Nishio K Facile and Highly Stereoselective Allylation of Aldehydes Using Allyltrichlorosilanes in DMF. Tetrahedron Lett 1993, 34, 3453. [Google Scholar]; (f) Denmark SE; Barsanti PA; Beutner GL; Wilson TW Enantioselective Ring Opening of Epoxides with Silicon Tetrachloride in the Presence of a Chiral Lewis Base. Mechanism Studies. Adv. Synth. Catal 2007, 349, 567. [DOI] [PubMed] [Google Scholar]; (g) Denmark SE; Eklov BM Neutral and Cationic Phosphoramide Adducts of Silicon Tetrachloride: Synthesis and Characterization of Their Solution and Solid-State Structures. Chem. - Eur. J 2008, 14, 234. [DOI] [PubMed] [Google Scholar]; (h) Bobbink FD; Menoud F; Dyson PJ Synthesis of Methanol and Diols from C02 via Cyclic Carbonates Under Metal-Free, Ambient Pressure, and Solvent-Free Conditions. ACS Sustainable Chem. Eng 2018, 6, 12119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


