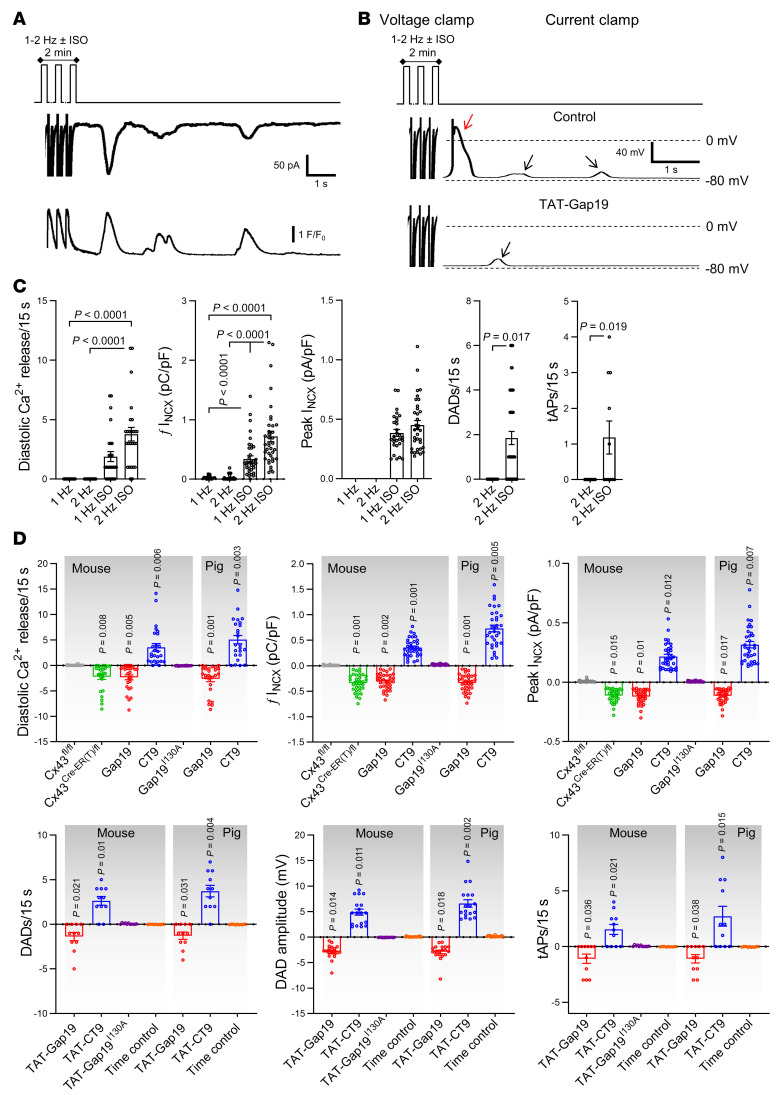
Figure 6. Cx43 hemichannel opening during adrenergic stimulation modulates spontaneous Ca2+ release from the sarcoplasmic reticulum and arrhythmogenic afterdepolarizations.
(A) Freshly isolated mouse and pig left ventricular cardiomyocytes were subjected to voltage clamp experiments while [Ca2+]i was simultaneously monitored. Top trace shows experimental protocol: cells were paced to steady state for 2 minutes at 1 Hz and then clamped to –70 mV. Middle and bottom traces depict resulting current and [Ca2+]i signals: final 3 paced Ca2+ transients and accompanying currents followed by 15-second rest period showing spontaneous diastolic Ca2+ release with resulting NCX current. Protocols were repeated at 2 Hz with and without isoproterenol. Example traces were recorded in pig cardiomyocytes. (B) In a subset of experiments, we switched to current clamp mode following steady-state pacing in voltage clamp. Example traces, recorded in the same pig cardiomyocyte, without and with TAT-Gap19 were recorded in current clamp mode following 2-minute pacing to steady state at 2 Hz with isoproterenol (in voltage clamp mode). Black arrows indicate DADs, red arrow indicates a triggered action potential. (C) Summary dot plots (nested 1-way ANOVA; N/nmouse = 23/75 for voltage clamp experiments and N/nmouse = 5/45 for current clamp experiments) illustrating increased frequency and amplitude of diastolic Ca2+ release with increased resulting NCX current and membrane depolarization during adrenergic stimulation (2 Hz + ISO) compared with baseline. tAP, triggered action potential. Similar results were obtained in pig cardiomyocytes (not shown). (D) Summary data showing the impact of different interventions on diastolic Ca2+ release and resulting NCX currents and membrane depolarization (nested 1-way ANOVA; N/nmouse = 5–11/15–24 per condition, N/npig = 5/15–20 per condition). Values reported as differences from the control condition.