Figure 5. MMP-14 and Prox-1 are required for lymphatic cord-like structures formation.
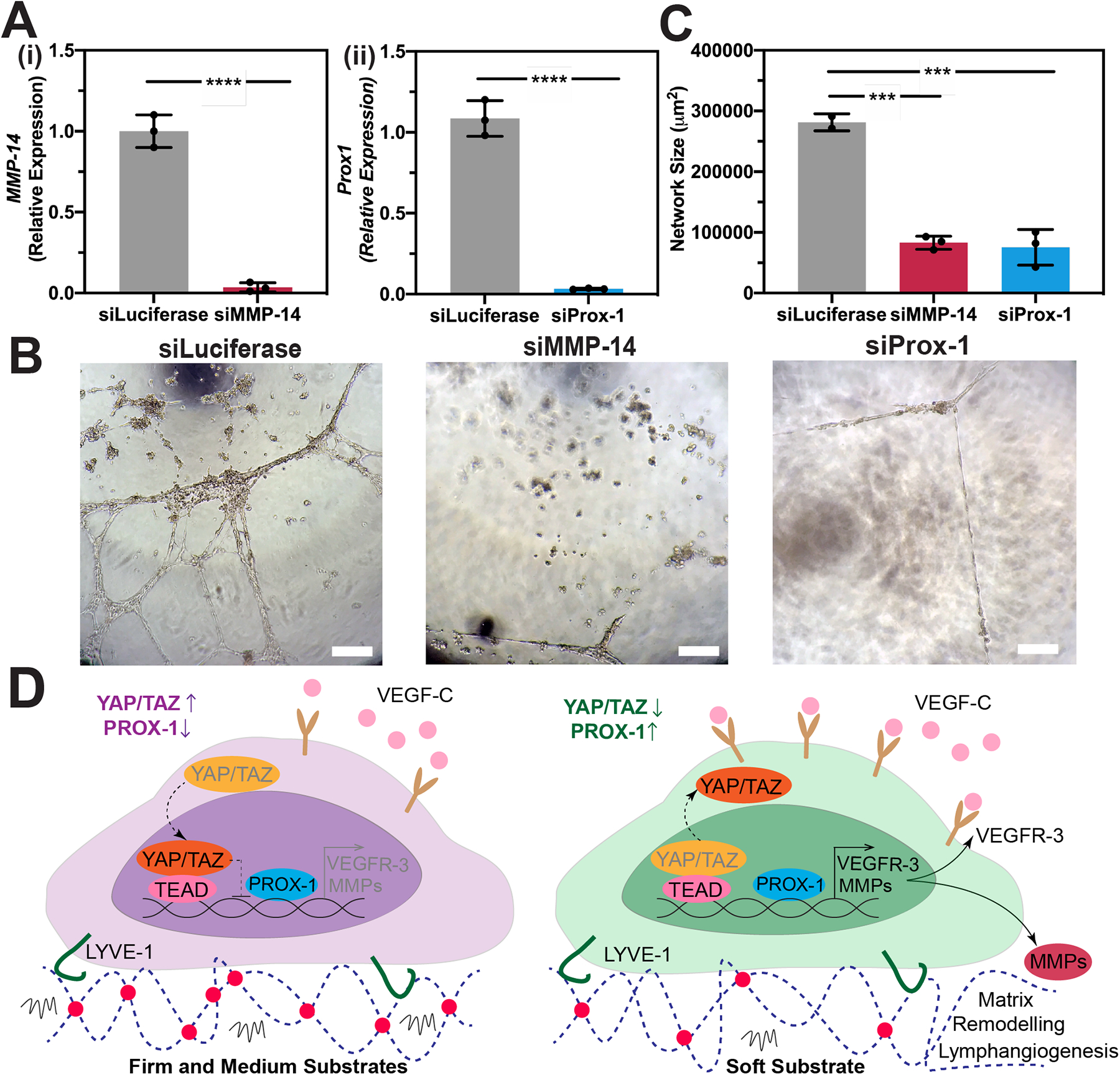
(A) Human LECs transfected with siRNA for (i) MMP-14 or (ii) Prox-1 demonstrated a significant reduction in their expression for MMP-14 or Prox-1, respectively compared to non-targeting control (siLuciferase). Three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings. Statistical significance was assessed using Paired Student’s t-test. Significance levels were set at ****P<0.001.(B) Human LECs transfected with either siLuciferase, siMMP-14, or siProx-1 were seeded on the soft substrates and supplemented with 50 ng/mL VEGF-C for 12 hrs. Scale bars are 50 μm. (C) Kinetic analysis of vasculogenesis (KAV) revealed a significant reduction in network size in siMMP-14 and siProx-1 treated groups compared to the siLuciferase control. Data represents the mean ± S.D. of three biological replicates performed. Statistical significance was assessed using Paired Student’s t-test to analyze differences between RNAi-treated groups and the Luciferase control. Significance levels were set at ***P<0.01. (D) Schematic diagram depicting the role of matrix elasticity in priming lymphatic tube formation directed by VEGF-C. HA-hydrogels were able to preserve key lymphatic markers, including LYVE-1. When LECs are cultured in firm and medium substrates, YAP/TAZ enter the nucleus and bind to the PROX-1 promoter, inhibiting its transcription, including its targets, such as VEGFR-3 and MMPs. However, decreasing matrix stiffness further primes LECs and enables YAP/TAZ to undergo cytoplasmic degradation, which subsequently enhance transcription of PROX-1, including its targets, such as VEGFR-3 and MMPs. Consequently, high MMP expression and binding of VEGF-C to VEGFR-3 results in matrix remodeling and lymphatic tube formation in vitro.
