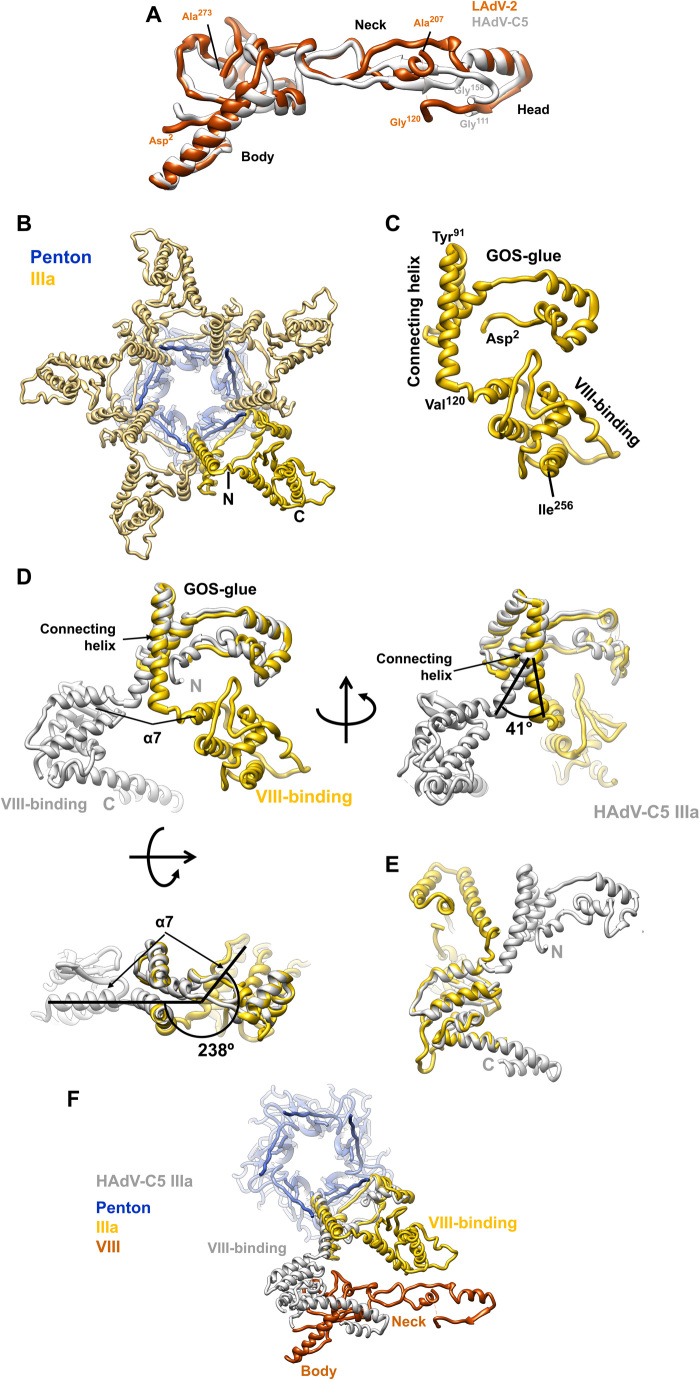
Fig. 6. LAdV-2 internal minor coat proteins: VIII and IIIa.
(A) Superposition of the LAdV-2 and HAdV-C5 protein VIII structures. The body, neck, and head domains are indicated, as well as the positions of the N- and C-terminal residues in the structure and the residues flanking the central gap. (B) A view from inside the capsid along a fivefold axis showing the ring of protein IIIa. One copy of IIIa is highlighted in vivid yellow. (C) Structure of the IIIa monomer. The GOS-glue and VIII-binding domains, as well as the connecting helix, the first and last residues traced, and those flanking the connecting helix are indicated. (D) Comparison between the LAdV-2 (yellow) and HAdV-C5 (gray) protein IIIa structures, presented in their original position in the capsid, in which the GOS-glue domains and part of the connecting helix overlap. Three points of view are shown, and angles between the connecting helices and between the first helix in the VIII-binding domain of each protein (α7) are indicated. (E) Superposition of the two VIII-binding domains showing the fold similarity. (F) LAdV-2 vertex proteins depicted together with HAdV-C5 protein IIIa, to show the effect of the large conformational change on its interactions with protein VIII.