Figure 5.
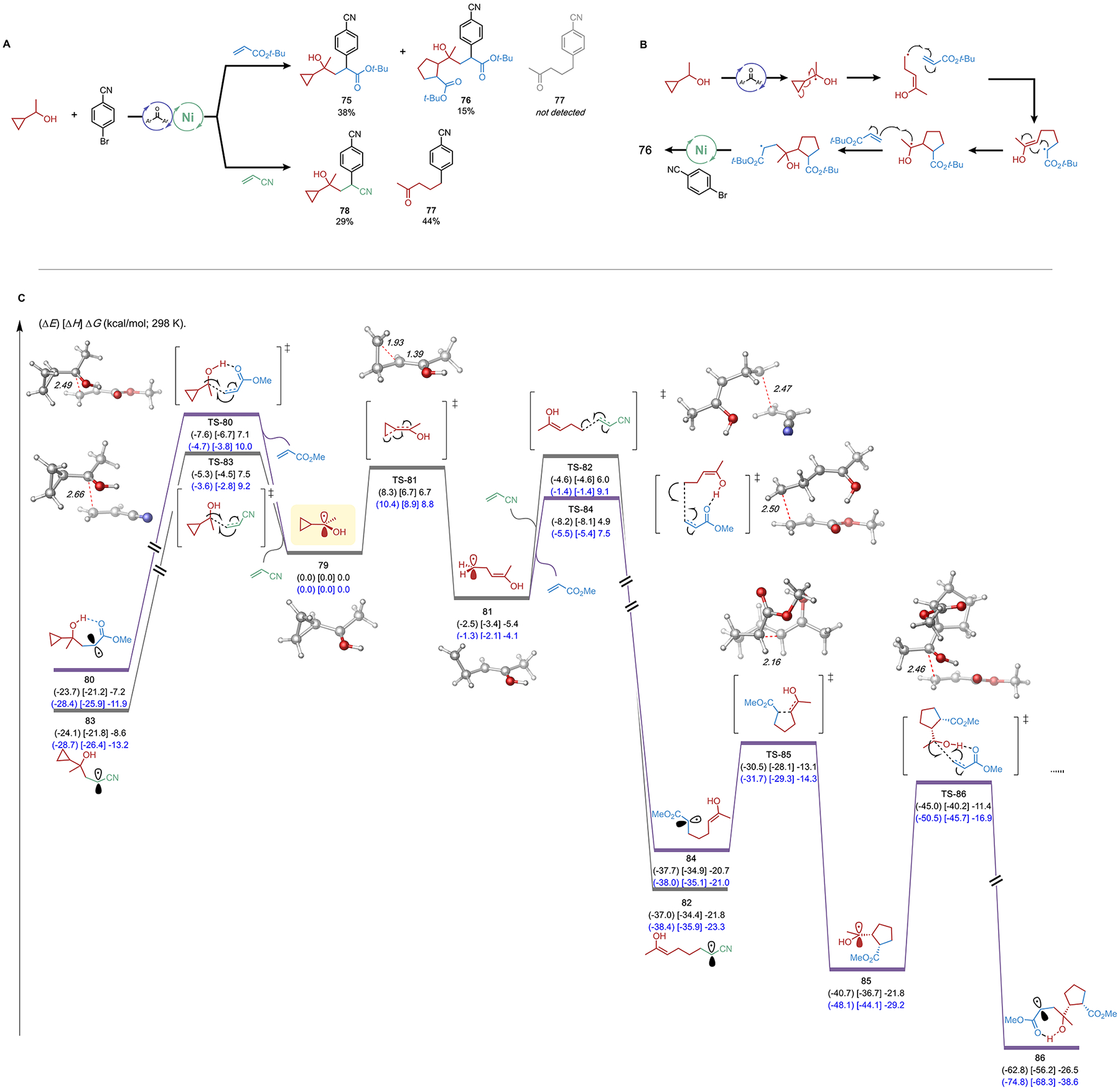
Experiments with 1-cyclopropylethanol as C–H precursor. (A) DCF reactions with 1-cyclopropylethanol; standard reaction conditions as in Table 1. (B) Proposed pathway of the formation of cyclopentane-containing DCF product. (C) Computational analysis of the competing ring-opening and Giese addition of tertiary radical in acrylate and acrylonitrile systems. Electronic (in parentheses), enthalpy (in bracket), and free energies (kcal/mol; 298 K) given were calculated at the (U)B3LYP-D3/def2-TZVPP-CPCM(benzene)//(U)B3LYP-D3/def2-SVP-CPCM-(benzene) (black) and DLPNO–CCSD(T)/def2-TZVPP//(U)B3LYP-D3/def2-SVP-CPCM(benzene) (blue) levels of method.
