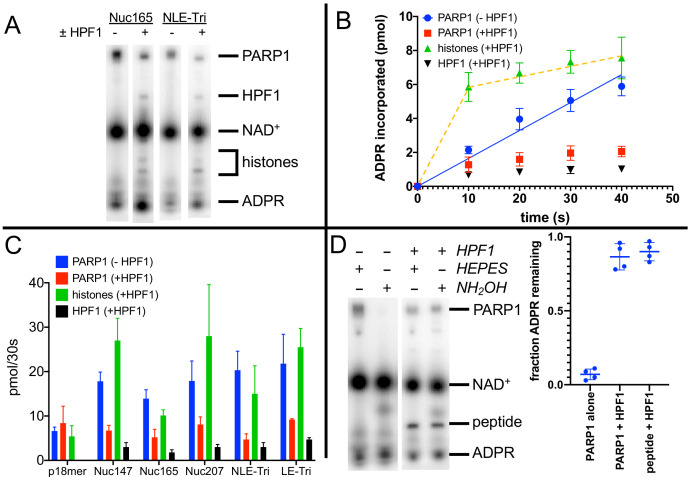
Figure 3. Histone PARylation Factor 1 (HPF1) mediates transPARylation onto nucleosomes and serines.
(A) Representative autoradiogram from an assay gel demonstrating the reduction of autoPARylation in the presence of HPF1 with the concomitant appearance of PARylated histones and HPF1. The remaining substrate 32P-NAD+ is by far the most prominent band on the image, indicating that we are monitoring the early time points of the reaction. (B) Time dependence of ADP-ribose (ADPR) incorporation onto poly(ADP-ribose) polymerase 1 (PARP1), histones, and HPF1. Note the linearity seen for autoPARylation in the absence of HPF1 and the non-linearity seen for PARylation in the presence of HPF1, as emphasized by the dotted line. (C) Bar graph of ADPR incorporation onto PARP1, histones, and HPF1 at 30 s demonstrating that in the presence of HPF1 histones become the primary target of PARylation to the detriment of PARP1. Levels of HPF1 PARylation are low despite the high concentration of HPF1 (2 µM) compared to nucleosomes (300–700 nM) in the reaction mixture. For the reaction indicated with p18mer, activation is by free DNA and the PARylated product is the H3 peptide. Each experiment was performed four separate times, and the data shown are mean values with standard deviations. (D) Representative gel demonstrating that PARylation in the absence of HPF1 is directed towards hydroxylamine labile Asp/Glu residues and in the presence of HPF1 becomes stable to this treatment, consistent with PARylation of Ser residues. Quantitation in the bar graph is the summary of 8–12 replicates.