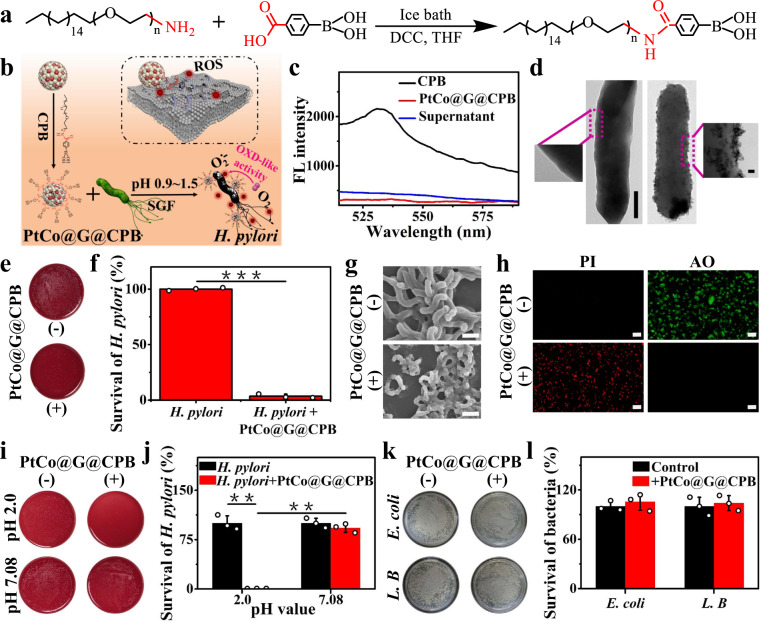
Fig. 2. C18-PEGn-benzeneboronic acid (CPB) synthesis and PtCo@G@CPB selective treatment of H. pylori in vitro.
a Schematic illustration of C18-PEGn-Benzeneboronic acid synthesis. b Schematic of CPB modified PtCo@G nanocrystals and antibacterial mechanism of PtCo@G@CPB against H. pylori. c Fluorescence spectra characterization of CPB functionalized PtCo@G. d TEM images showing the ability of PtCo@G@CPB to target H. pylori. H. pylori incubated with PtCo@G (left) and PtCo@G@CPB (right) at SGF for 0.5 h. Scale bar: left 0.5 μm, right 20 nm. e Photographs of H. pylori colonies. f The antibacterial activity of PtCo@G@CPB against H. pylori. P values were calculated by the Student’s two-sided t-test: ***p < 0.001. g SEM images of H. pylori treatment without or with PtCo@G@CPB. Scale bar: 1.0 μm. h Fluorescence images of live (green) and dead (red) H. pylori after incubation without or with PtCo@G@CPB. PI: propidium iodide, AO: acridine orange. Scale bar: 10 μm. i Photographs of H. pylori colonies under various conditions. j Relative bactericidal activity of PtCo@G@CPB against H. pylori under gastric acid environment and neutral intestinal environment. P values were calculated by the Student’s two-sided t-test: **p < 0.01. k Photographs of E. coli and L. B colonies under different conditions. l The relative antibacterial activity of PtCo@G@CPB against intestinal symbiotic bacteria. The concentration of PtCo@G@CPB used in the above antibacterial experiments was 70 μg/mL. The data indicate the means and SD from three parallel experiments. Source data are provided as a Source Data file.