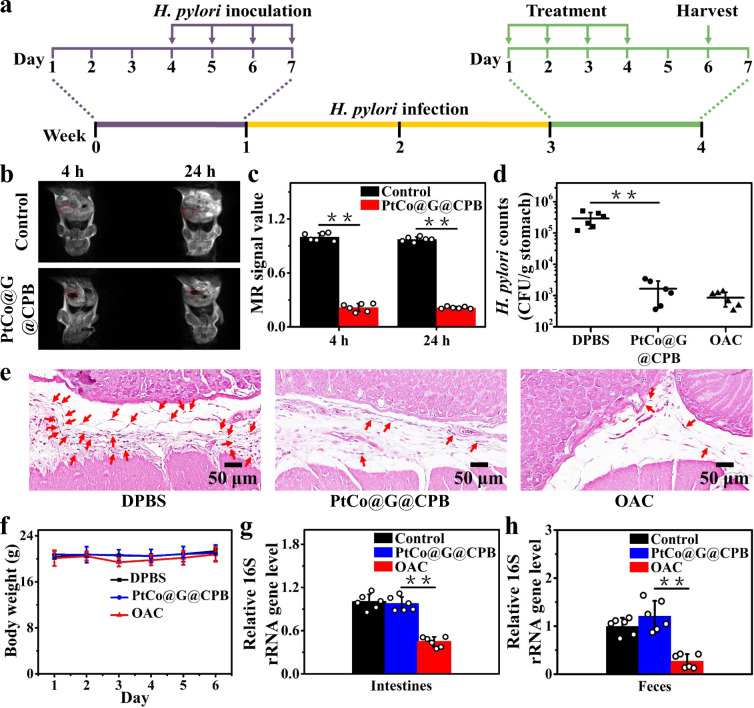
Fig. 4. In vivo therapeutic effect of PtCo@G@CPB.
a The study protocol of H. pylori inoculation, infection development, and treatment in BALB/c mice. b Retention time of PtCo@G@CPB in the stomach of H. pylori-infected mouse. c Corresponding data analysis of mouse stomach in b. Data are presented as mean ± SD (n = 6 biologically independent mice). P values were calculated by the Student’s two-sided t-test: **p < 0.01. d Bacterial burden in the stomach of H. pylori-infected mice treated with DPBS, PtCo@G@CPB, and triple therapy. Data are presented as mean ± SD (n = 6 biologically independent mice). P values were calculated by the Student’s two-sided t-test: **p < 0.01. e Gram staining of slice from the gastric mucosa receiving DPBS, PtCo@G@CPB, and OAC treatments. Red arrows point to bacteria. f Bodyweight change of mice following treatment of various formulations as in d. Data are presented as mean ± SD (n = 6 biologically independent mice). The side effects of PtCo@G@CPB against symbiotic bacteria in g intestine and h feces of mice. Data are presented as mean ± SD (n = 6 biologically independent mice). P values were calculated by the Student’s two-sided t-test: **p < 0.01. Source data are provided as a Source Data file.