Abstract
Background
During the current coronavirus disease 2019 (COVID‐19) pandemic, the cytopathology workload has decreased remarkably worldwide as all screening and elective procedures have been postponed to prioritize the clinical management of patients at high oncological risk. In the current study, the authors provide data on the lasting impact of COVID‐19 on cytopathology practice during the initial phases of the Italian postlockdown period.
Methods
The percentages of the cytological sample types processed at the University of Naples Federico II during the first 12 weeks of the Italian postlockdown period were compared with those of the same period in 2019. The study period was divided into four 3‐week periods. Differences in the rates of malignant diagnoses were also assessed.
Results
During the 12‐week study period, the overall cytological sample workload decreased by 41.6% in comparison with 2019. In particular, the workload significantly declined for each sample type: Pap smears, –33.3%; urine, –42.8%; serous fluids, –14.4%; thyroid, –54.5%; breast, –43%; lymph node, –27.3%; and salivary gland, –61%. By contrast, the overall malignancy rate was significantly increased (P = .0011).
Conclusions
The reduction in the cytological sample workload during the postlockdown period still represents an ongoing effect of the COVID‐19 pandemic. On the other hand, the rise in the overall malignancy rate reflects the importance of prioritizing diagnostic procedures for patients at high oncological risk.
Keywords: cancer, coronavirus disease 2019 (COVID‐19), cytopathology, fine‐needle aspiration (FNA), screening programs
Short abstract
The reduction in the cytological sample workload during the postlockdown period represents an ongoing effect of the COVID‐19 pandemic. On the other hand, the rise in the overall malignancy rate reflects the importance of prioritizing diagnostic procedures for patients at high oncological risk.
Introduction
The current coronavirus disease 2019 (COVID‐19) pandemic has induced major changes in cytopathological practice. 1 , 2 Indeed, this past spring, many of the worst hit countries, including Italy, were subjected to national lockdowns to limit the spread of the disease. This resulted in a substantial reduction in outpatient care practices. Among these, fine‐needle aspiration (FNA) clinics saw a relevant drop in the number of processed samples. 1 , 2 , 3 , 4 , 5 Furthermore, because of the potential presence of the virus in cytological specimens, a number of laboratory biosafety guidelines recommended that the risks of contagion be carefully weighed against the clinical benefits of any diagnostic procedure. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 These factors markedly reduced cytopathology workloads worldwide. In Italy, the activity of cytopathology practice also declined. For instance, our laboratory workload decreased by more than 80% during the first 3 weeks of the lockdown in comparison with the same period in 2019. 5 During this emergency, our laboratory performed only urgent activities to comply with the COVID‐19 containment measures. For instance, breast and lymph node FNA specimens were prioritized over thyroid FNA, whereas serous fluid and urine specimens were prioritized over Papanicolaou (Pap) smears. However, to mitigate any secondary effects in terms of changes in the prevalence and mortality rate of neoplastic diseases, all screening procedures must be ensured as soon as possible. 14
On May 4, 2020, the Italian government eased safety measures; this began the so‐called postlockdown period. In those days, at the University of Naples Federico II, the rate of positivity in COVID‐19 testing dropped to 1%, and this led the hospital managers to reopen all the wards closed by COVID‐19. The purpose of this study was, therefore, to verify whether our cytopathology laboratory activity actually returned to normal in the initial phases of the postlockdown period. For this purpose, we compared our workload activity from May 4 to July 27, 2020, with our workload for the corresponding period in 2019.
Materials and Methods
All cytological reports issued from May 4 to July 27, 2020, were examined and compared with those recorded in the same period in 2019. All information regarding human material was managed using anonymous numerical codes, and all samples were handled in compliance with the Helsinki Declaration. The data were grouped into four 3‐week periods. Period I was from May 4 to May 24, period II was from May 25 to June 14, period III was from June 15 to July 5, and period IV was from July 6 to July 27. The overall workload rates for each specimen type were compared, and the total number of processed samples was recorded. In particular, the numbers of Pap smears and urine and serous fluid samples were calculated. Similarly, the total number of thyroid, breast, lymph node, and salivary gland ultrasound‐guided FNAs was detailed. Sample sites with fewer than 20 specimens were grouped in an “other” category.
Differences between the postlockdown period and the corresponding 2019 period were evaluated on the basis of absolute numbers and proportional changes (the percentage of the total workload) for each specimen type via the χ2 test; P values lower than .05 were deemed to be statistically significant. Differences in the rates of malignant diagnoses were assessed in a similar way.
Results
During the 12‐week postlockdown study period (from May 4 to July 27, 2020), among cytopathology laboratory staff, no COVID‐19 cases were reported, and the FNA clinic was run by 2 cytopathologists, 4 days a week, as usual. Overall, fewer cytological samples (n = 1367) were processed in comparison with the corresponding period in 2019 (n = 2341), with a total reduction of 41.6%. The reduction was consistently evident in each of the 4 postlockdown periods (period I: 200 vs 581, –66%; period II, 282 vs 575, –51%; period III, 462 vs 614, –25%; period IV, 423 vs 571, –26%; Table 1). Accordingly, all the different specimen types showed drastic reductions, which ranged from –14.4% (serous fluids) to –61% (salivary gland; Table 2).
TABLE 1.
Cytological Sample Workload During the 12‐Week Postlockdown Period Versus the Corresponding Period in 2019
| Total Samples, No. | Difference, % | ||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | ||
| Period I | 200 | 581 | –66 |
| Period II | 282 | 575 | –51 |
| Period III | 462 | 614 | –25 |
| Period IV | 423 | 571 | –26 |
| Total | 1367 | 2341 | –41.6 |
Abbreviation: COVID‐19, coronavirus disease 2019.
Data are grouped into four 3‐week periods: period I (May 4 to May 24), period II (May 25 to June 14), period III (June 15 to July 5), and period IV (July 6 to July 27).
TABLE 2.
Overall Number and Proportion of Samples From Each Sample Site During the First 12 Weeks of the Postlockdown Period and the Corresponding Period in 2019
| Sample Site | Overall | Proportion | ||||
|---|---|---|---|---|---|---|
| COVID‐19 Postlockdown, No. | Corresponding Period in 2019, No. | Difference, % | COVID‐19 Postlockdown, % | Corresponding Period in 2019, % | P | |
| Pap smear | 542 | 813 | –33.3 | 39.6 | 34.7 | .0027 |
| Urine | 135 | 236 | –42.8 | 9.9 | 10.1 | .84 |
| Serous fluids | 113 | 132 | –14.4 | 8.3 | 5.6 | .0019 |
| Thyroid | 397 | 872 | –54.5 | 29 | 37.2 | 0 |
| Breast | 61 | 107 | –43 | 4.5 | 4.6 | .87 |
| Lymph node | 56 | 77 | –27.3 | 4.1 | 3.3 | .2 |
| Salivary gland | 23 | 59 | –61.1 | 1.7 | 2.5 | .09 |
| Other | 40 | 45 | –11.1 | 2.9 | 1.9 | .05 |
Abbreviations: COVID‐19, coronavirus disease 2019; Pap, Papanicolaou.
Granular data analysis revealed different trends for each specimen type. Overall, during the postlockdown period, Pap smear, urine, thyroid, and salivary gland workloads decreased in each of the four 3‐week periods (Table 3 and Fig. 1).
TABLE 3.
Overall Number of Each Cytological Sample Type Grouped Into Four 3‐Week Periods During the COVID‐19 Postlockdown Period and the Corresponding Period in 2019
| Pap Smear | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 75 | 196 | –61 |
| Period II | 120 | 191 | –37.2 |
| Period III | 190 | 217 | –12.4 |
| Period IV | 157 | 209 | –24.9 |
| Urine | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 25 | 68 | –63 |
| Period II | 47 | 68 | –30 |
| Period III | 46 | 52 | –11.5 |
| Period IV | 17 | 48 | –64.6 |
| Serous Fluid | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 26 | 26 | 0 |
| Period II | 23 | 38 | –39.5 |
| Period III | 32 | 43 | –25.6 |
| Period IV | 32 | 25 | +28 |
| Thyroid | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 40 | 238 | –83 |
| Period II | 60 | 196 | –69.4 |
| Period III | 146 | 230 | –36.5 |
| Period IV | 151 | 208 | –27.4 |
| Breast | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 9 | 26 | –65 |
| Period II | 6 | 27 | –77 |
| Period III | 19 | 29 | –34.5 |
| Period IV | 27 | 25 | +8 |
| Lymph Node | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 14 | 10 | +40 |
| Period II | 13 | 26 | –50 |
| Period III | 15 | 18 | –16.7 |
| Period IV | 14 | 23 | –39.1 |
| Salivary Gland | |||
|---|---|---|---|
| COVID‐19 Postlockdown | Corresponding Period in 2019 | Difference, % | |
| Period I | 7 | 10 | –30 |
| Period II | 2 | 19 | –89.5 |
| Period III | 5 | 13 | –61.5 |
| Period IV | 9 | 17 | –47 |
Abbreviations: COVID‐19, coronavirus disease 2019; Pap, Papanicolaou.
The data are grouped into period I (May 4 to May 24, 2020), period II (May 25 to June 14, 2020), period III (June 15 to July 5, 2020), and period IV (July 6 to July 27, 2020).
Figure 1.
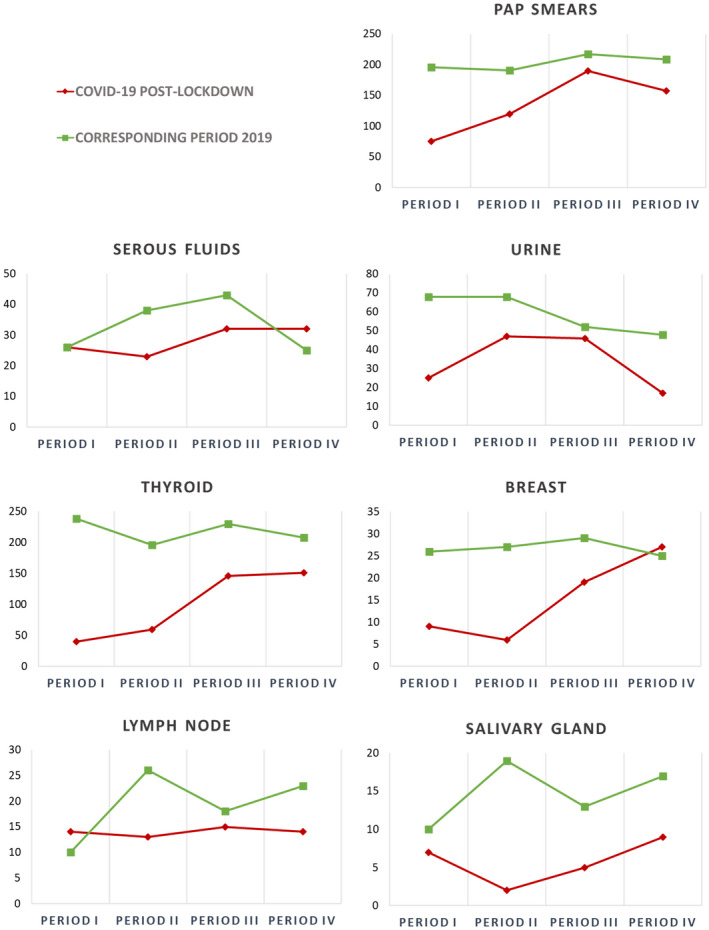
Line charts of the workload for each cytological sample type grouped into four 3‐week periods during the COVID‐19 postlockdown and the corresponding period in 2019: period I (May 4 to May 24, 2020), period II (May 25 to June 14, 2020), period III (June 15 to July 5, 2020), and period IV (July 6 to July 27, 2020). COVID‐19 indicates coronavirus disease 2019; Pap, Papanicolaou.
As for the ratio between the different types of specimens and the overall activity (the percentage of the total workload), thyroid samples significantly decreased (29% vs 37.2%), whereas Pap smears (39.6% vs 34.7%) and serous fluid samples (8.3% vs 5.6%) increased. On the other hand, no significant proportional variations were observed in urine, breast, lymph node, or salivary gland specimens (Table 2). Detailed data for each 3‐week period are reported in Supporting Table 1.
The overall malignancy rate of the total samples obtained during the whole study period showed a statistically significant increase (127 [9.3%] vs 149 [6.4%]; P = .0011). A detailed analysis of all four 3‐week periods showed a significant increase in the malignancy rates recorded in period I (14% vs 6.2%; P = .0005), with relatively similar rates in periods II (9.6% vs 6.1%; P = .06), III (6.9% vs 6.8%; P = .95), and IV (9.5% vs 6.3%; P = .064; Table 4).
TABLE 4.
Malignancy Rates During the Postlockdown Versus the Corresponding Period in 2019
| COVID‐19 Postlockdown, % | Corresponding Period in 2019, % | P | |
|---|---|---|---|
| 12 wk | 9.3 | 6.4 | .0011 |
| Period I | 14 | 6.2 | .0005 |
| Period II | 9.6 | 6.1 | .06 |
| Period III | 6.9 | 6.8 | .95 |
| Period IV | 9.5 | 6.3 | .064 |
Abbreviation: COVID‐19, coronavirus disease 2019.
Data are reported as overall values for the entire study period (12 weeks) and for each 3‐week period. In bold statistically significant P values.
Discussion
Cytopathology plays a key role in the diagnosis and management of human tumors. Unfortunately, since the beginning of the current health crisis, restrictive measures, imposed to thwart the relentless spread of COVID‐19, have dramatically disrupted many health care services, not least cytopathology practices. 1 , 2 Although the Italian government eased safety measures on May 4, 2020, the impact of the coronavirus emergency persisted beyond the end of the lockdown. Indeed, our clinic experienced an overall workload reduction of 41.6% in the first weeks of the postlockdown. In particular, Pap smears dropped by 33.3%, urine specimens dropped by 42.8%, and serous fluids dropped by 14.4%. Likewise, thyroid FNA declined by 54.5%, breast FNA declined by 43%, lymph node FNA declined by 27.3%, and salivary gland FNA declined by 61.1%. These data are greatly worrisome because delayed diagnoses can lead to worse clinical outcomes.
It was encouraging to see that in periods III (–25%) and IV (–26%), our workload reduction was not as dramatic as it was in periods I (–66%) and II (–51%); this suggests that a gradual return to prelockdown workload levels for screening and elective activities also might be foreseen. In particular, in periods III and IV, Pap smears increased in terms of absolute numbers and relative proportions. As for thyroid FNA, it was not surprising to see that in period I, these procedures decreased by a whopping 83%. This phenomenon is explainable by the fact that thyroid FNA, which generally falls into the category of nonurgent and elective procedures, was suspended to contain contagion. 3 , 5 , 15 , 16 , 17 However, this trend was inverted in the later periods of the postlockdown. Indeed, in period IV, thyroid FNA practice almost returned to normal, with a workload reduction of only 27.4% in comparison with the previous year. As with thyroid FNA, breast FNA also increased during the postlockdown (–65% in period I and +8% in period IV). However, this increase was nonlinear; indeed, fewer FNA procedures were performed in period II than period I. It is conceivable that the higher number of patients subjected to FNA in period I was due to the fact that these patients had been selected before the lockdown. On the other hand, the lower number of FNA procedures in period II was ascribable to the recent resumption of routine cancer detection programs. Reassuringly, the positive trend observed in period IV confirmed the role of breast FNA as a valuable component of the triple test, especially in the era of COVID‐19. 18 , 19 A similar nonlinear trend was also reported for salivary gland FNA. Indeed, the FNA workload reduction was more evident in periods II (–89.5%) and III (–61.5%) than periods I (–30%) and IV (–47%).
Conversely, samples at high oncological risk, such as serous fluids and lymph node FNA, showed a steady trend during the different postlockdown periods. These samples were only slightly reduced in comparison with the prelockdown period; this phenomenon probably reflected the attitudes of cytopathologists toward the timely evaluation of FNA specimens even in the midst of a global health crisis. 2 A fluctuating trend was finally observed for urine samples. In these cases, a more evident reduction was observed in periods I and IV versus periods II and III; this trend probably reflected an inherent variability in the sample workload. Indeed, regardless of COVID‐19–related effects, the cytopathology workload is influenced by a number of additional factors; for example, in 2019, the total sample number was lower in period IV, and this probably reflected a seasonal variation on the eve of summer holidays.
Overall, the data that we collected and processed during the initial weeks of the postlockdown period clearly indicated that the return to regular cytology practice was challenging. Indeed, a workload reduction was observed for all specimen types. However, as already seen in the lockdown period, the prioritization policy partially mitigated the negative impact of COVID‐19 on health care services. 2 , 5 In fact, a significant increase in the overall malignancy rate was observed, and this suggests that special care was taken to diagnose high‐risk oncological patients. Interestingly, a data subanalysis showed that the rise in malignant specimens occurred mainly in period I (Table 4). Indeed, our clinic continued to prioritize suspicious cases at the very beginning of the postlockdown period in an effort to limit the diagnostic delay. This was rendered possible by the close interaction between cytopathologists and clinicians, which was ensured by timely, web‐based multidisciplinary meetings. 11 Because most countries suffer from increased medical expenses, a lesson that must be learned in this health emergency is the need to properly select patients for cytopathology evaluations to prevent overtesting and limited cost‐effectiveness. Conversely, the workload of predictive molecular testing performed on cytological samples showed little variation, and this allowed timely selection of advanced‐stage oncological patients for targeted treatments. 20 , 21 On the other hand, a proportional reduction of local treatment (surgery or radiation) for patients with early‐stage cancer could have represented an aftermath of the risk/benefit ratio in treatment decisions. 22
Overall, despite the increase in the malignancy rate, few patients underwent cytopathology workup, and the identification of patients at high oncological risk remained insufficient even several weeks after the lockdown. In this regard, we speculate that the limitations imposed by our national health care system and the persistent reluctance of frail older patients to go to the hospital, even when many restrictions were lifted, constituted 2 effects of the pandemic that, albeit unquantifiable, indirectly contributed to reducing our cytology workload. We thus maintain that the data reported in this study should be viewed not as solely academic but rather as a reflection of present‐day reality. Indeed, nowadays, as the world is trying to cope with a new wave of COVID‐19, all efforts should be made to prevent any further decline in the cytological workload for the sake of cancer prevention and the early diagnosis of high‐risk cancer patients. 14 , 23 In this setting, we may reasonably hope that more significant changes, with a return to prepandemic levels of cytopathology practice, will soon take place because of the implementation of mass vaccination campaigns.
In conclusion, we have shown that the overall reduction in cytological specimen volume in the postlockdown period is a lingering effect of the ongoing COVID‐19 pandemic. However, the increase in the overall malignancy rate during the initial period of the postlockdown illustrates that the prioritization of patients at high oncological risk is key to ensuring that these patients do not miss the opportunity to benefit from the necessary preventive and diagnostic care, even in the midst of a pandemic. Finally, collaborative, multi‐institutional studies are required to monitor the effects of COVID‐19 on cytopathology worldwide.
Funding Support
This study was supported by Monitoraggio Ambientale, Studio ed Approfondimento della Salute della Popolazione Residente in Aree a Rischio–In Attuazione della D.G.R. Campania n.180/2019, POR Campania FESR 2014‐2020 Progetto “Sviluppo di Approcci Terapeutici Innovativi per Patologie Neoplastiche Resistenti ai Trattamenti–SATIN,” and the Campania Region for the Investigation of the Molecular Biology of Thyroid Cancer (grant LR n.24 29/12/2005).
Conflict of Interest Disclosures
Elena Vigliar received personal fees as an advisor from Diaceutics for work unrelated to the current study. Umberto Malapelle received personal fees (as a member of a speakers' bureau or an advisor) from Boehringer Ingelheim, AstraZeneca, Roche, MSD, Amgen, and Merck for work unrelated to the current study. Giancarlo Troncone received personal fees (as a member of a speakers' bureau or an advisor) from Roche, MSD, Pfizer, and Bayer for work unrelated to the current study. The other authors made no disclosures.
Author Contributions
Elena Vigliar: Conceptualization, software and formal analysis, methodology, validation, investigation, resources, data curation, visualization, supervision, project administration, writing–original draft preparation, and writing–review and editing. Rima Cepurnaite: Software and formal analysis, methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Antonino Iaccarino: Methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Pasquale Pisapia: Methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Caterina De Luca: Methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Umberto Malapelle: Methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Claudio Bellevicine: Methodology, validation, investigation, resources, data curation, visualization, and writing–review and editing. Giancarlo Troncone: Conceptualization, methodology, validation, investigation, resources, data curation, visualization, supervision, project administration, funding acquisition, writing–original draft preparation, and writing–review and editing.
Supporting information
Table S1
Vigliar E, Cepurnaite R, Iaccarino A, Pisapia P, De Luca C, Malapelle U, Bellevicine C, Troncone G. Cytopathology practice during the COVID‐19 postlockdown: An Italian experience. Cancer Cytopathol. 2021. 10.1002/cncy.22416
We thank Paola Merolla for English language editing.
References
- 1. Wang Y, Bychkov A, Chakrabarti I, et al. Impact of the COVID‐19 pandemic on cytology practice: an international survey in the Asia‐Pacific region. Cancer Cytopathol. 2020;128:895‐904.doi: 10.1002/cncy.22354 [DOI] [PubMed] [Google Scholar]
- 2. Vigliar E, Cepurnaite R, Alcaraz‐Mateos E, et al. Global impact of the COVID‐19 pandemic on cytopathology practice: results from an international survey of laboratories in 23 countries. Cancer Cytopathol. 2020;128:885‐894.doi: 10.1002/cncy.22373 [DOI] [PubMed] [Google Scholar]
- 3. Rana C, Kumar S, Babu S, et al. Impact of ongoing COVID‐19 pandemic on cytology: an institutional experience. Diagn Cytopathol. 2021;49:311‐315.doi: 10.1002/dc.24620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Pelsemaeker MC, Guiot Y, Vanderveken J, Galant C, Van Bockstal MR. The impact of the COVID‐19 pandemic and the associated Belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology. 2021;88:46‐55.doi: 10.1159/000509546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigliar E, Iaccarino A, Bruzzese D, Malapelle U, Bellevicine C, Troncone G. Cytology in the time of coronavirus disease (COVID‐19): an Italian perspective. J Clin Pathol. Published online April 20, 2020. doi: 10.1136/jclinpath-2020-206614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbareschi M, Ascoli V, Bonoldi E, et al. Biosafety in surgical pathology in the era of SARS‐Cov2 pandemia. A statement of the Italian Society of Surgical Pathology and Cytology. Pathologica. 2020;112:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. College of American Pathologists . Cytopathology laboratory considerations during the COVID‐19 pandemic: College of American Pathologists Cytopathology Committee. Accessed July 29, 2020. https://www.cap.org/laboratory‐improvement/news‐and‐updates/cytopathology‐laboratory‐considerations‐during‐the‐covid‐19‐pandemic
- 8. Royal College of Pathologists , Institute of Biomedical Science , Association of Clinical Biochemistry and Laboratory Medicine , Association of Clinical Pathologists . Recommendations from RCPath and professional bodies (IBMS, ACP and ACB): prioritisation/deferral of pathology laboratory work (in light of SARS‐CoV‐2 (COVID19) epidemic). Accessed September 21, 2020. https//www.rcpath.org/uploads/assets/f5123842‐950f‐49c5‐bf69ed866a7ca3da/Prioritisation‐deferral‐of‐pathology‐laboratory‐work.pdf
- 9. Rossi ED, Fadda G, Mule A, Zannoni GF, Rindi G. Cytologic and histologic samples from patients infected by the novel coronavirus 2019 SARS‐CoV‐2: an Italian institutional experience focusing on biosafety procedures. Cancer Cytopathol. 2020;128:317‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baskota SU, Chandra A, Cross P. The practice of cytopathology during the era of COVID‐19: challenges and changes. Diagn Histopathol (Oxf). Published online October 12, 2020. doi: 10.1016/j.mpdhp.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pambuccian SE. The COVID‐19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol. 2020;9:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C, Chi C. Biosafety in the preparation and processing of cytology specimens with potential coronavirus (COVID‐19) infection: perspectives from Taiwan. Cancer Cytopathol. 2020;128:309‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gosney JR, Hofman P, Troncone G, Lopez‐Rios F. Cellular pathology in the COVID‐19 era: a European perspective on maintaining quality and safety. J Clin Pathol. 2021;74:64‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellevicine C, Vigliar E, Troncone G. Thyroid FNA in the time of coronavirus: the interventional cytopathologist point of view. Cancer Cytopathol. 2020;128:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troncone G. Thyroid cytology in the times of coronavirus. Diagn Cytopathol. Published online June 1, 2020. doi: 10.1002/dc.24510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palladino R, Migliatico I, Sgariglia R, et al. Thyroid fine‐needle aspiration trends before, during, and after the lockdown: what we have learned so far from the COVID‐19 pandemic. Endocrine. 2021;71:20‐25.doi: 10.1007/s12020-020-02559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinto D, Schmitt F. The role of breast fine needle aspiration during and post‐COVID‐19 pandemic: a fast and safe alternative to needle core biopsy. Cytopathology. 2000;31:627‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dinmohamed AG, Cellamare M, Visser O, et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID‐19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malapelle U, De Luca C, Iaccarino A, et al. Predictive molecular pathology in the time of COVID‐19. J Clin Pathol. Published online May 19, 2020. doi: 10.1136/jclinpath-2020-206711 [DOI] [PubMed] [Google Scholar]
- 21. Malapelle U, Pisapia P, Iaccarino A, et al. Predictive molecular pathology in the time of coronavirus disease (COVID‐19) in Europe. J Clin Pathol. Published online July 31, 2021. doi: 10.1136/jclinpath-2020-206957 [DOI] [PubMed] [Google Scholar]
- 22. European Society for Medical Oncology . Cancer patient management during the COVID‐19 pandemic. Accessed January 16, 2021. https://www.esmo.org/guidelines/cancer‐patient‐management‐during‐the‐covid‐19‐pandemic
- 23. Vose JM. Delay in cancer screening and diagnosis during the COVID‐19 pandemic: what is the cost? Oncology (Williston Park). 2020;34:343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


