FIGURE 1.
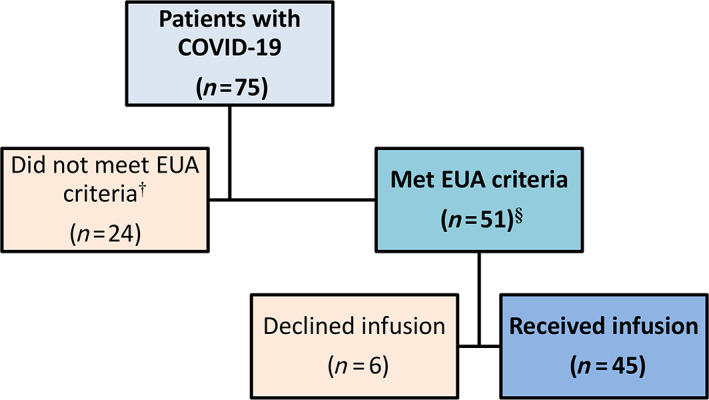
Allocation of monoclonal antibody to skilled care facility residents with coronavirus disease‐19. §Criteria for monoclonal antibody therapy under the FDA Emergency Use Authorization (EUA) include mild to moderate coronavirus disease‐19 (COVID‐19) within 10 days of onset of symptoms. Patients should be 65 years and older, have diabetes mellitus, have body mass index of 35 and higher, have chronic kidney disease, or have an immunocompromised status. Patients who are 55 years and older are also eligible if they have hypertension, chronic lung disease, or cardiovascular disease. See Table 1 for the distribution of risk profile of 45 patients who received the monoclonal antibody infusion. †Twenty‐four patients were not eligible for monoclonal antibodies because of advanced directives (hospice, comfort care, do‐not‐hospitalize status) (n = 8; 33.3%), asymptomatic infection (n = 3; 12.5%), severe COVID‐19 symptoms, as manifested by increasing oxygen requirement (n = 5; 20.8%), symptoms longer than 10 days (n = 3; 12.5%), or hospitalization for COVID‐19 (n = 5; 20.8%)
