Abstract
Since December 2019, the new coronavirus (also known as severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2, 2019‐nCoV])—induced disease, COVID‐19, has spread rapidly worldwide. Studies have reported that the traditional Chinese medicine Salvia miltiorrhiza possesses remarkable antiviral properties; however, the anti‐coronaviral activity of its main components, salvianolic acid A (SAA), salvianolic acid B (SAB), and salvianolic acid C (SAC) is still debated. In this study, we used Cell Counting Kit‐8 staining and flow cytometry to evaluate the toxicity of SAA, SAB, and SAC on ACE2 (angiotensin‐converting enzyme 2) high‐expressing HEK293T cells (ACE2h cells). We found that SAA, SAB, and SAC had a minor effect on the viability of ACE2h cells at concentrations below 100 μM. We further evaluated the binding capacity of SAA, SAB, and SAC to ACE2 and the spike protein of 2019‐nCoV using molecular docking and surface plasmon resonance. They could bind to the receptor‐binding domain (RBD) of the 2019‐nCoV with a binding constant (K D) of (3.82 ± 0.43) e−6 M, (5.15 ± 0.64)e−7 M, and (2.19 ± 0.14)e‐6 M; and bind to ACE2 with K D (4.08 ± 0.61)e−7 M, (2.95 ± 0.78)e−7 M, and (7.32 ± 0.42)e−7 M, respectively. As a result, SAA, SAB, and SAC were determined to inhibit the entry of 2019‐nCoV Spike pseudovirus with an EC50 of 11.31, 6.22, and 10.14 μM on ACE2h cells, respectively. In conclusion, our study revealed that three Salvianolic acids can inhibit the entry of 2019‐nCoV spike pseudovirus into ACE2h cells by binding to the RBD of the 2019‐nCoV spike protein and ACE2 protein.
Keywords: 2019‐nCoV, ACE2, salvianolic acid A, salvianolic acid B, salvianolic acid C
1. INTRODUCTION
Since December 2019, coronavirus disease (COVID‐19) has been affecting global health and thus far, there has been no sign of the pandemic subsiding. As of August 28, 2020, more than 10 million people had been infected, with 830,057 deaths recorded worldwide. 1 , 2 Patients infected with novel coronavirus (2019‐nCoV; also known as severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]) manifest many symptoms, such as dry cough, fever, headache, dyspnea, and pneumonia, with an estimated mortality rate ranging from 3% to 5%, which is lower than that in individuals infected with pathogenic SARS‐CoV (2002–2003) and Middle East respiratory syndrome coronavirus (MERS‐CoV, 2012). 3 , 4 , 5 Therefore, it is urgent to develop an effective program for the prevention and treatment of COVID‐19‐associated pneumonia.
2019‐nCoV is a positive‐sense single‐stranded RNA virus with a crown‐like appearance due to the projection of surface spike glycoproteins (S proteins) from its envelope. 6 Coronaviruses use the homotrimeric S protein (comprised of an S1 subunit and S2 subunit in each spike monomer) on the envelope to bind to their cellular receptors. 6 This binding triggers a cascade of events that lead to the fusion of the cell and viral membranes, enabling entry into the cell. As with SARS‐CoV, the receptor binding domain (RBD) of the 2019‐nCoV S protein binds to the angiotensin‐converting enzyme 2 (ACE2) cell receptor. 7 , 8 , 9 However, the RBD of 2019‐nCoV has a higher binding affinity for ACE2 than that of SARS‐CoV. 6 RBDs of S proteins of SARS‐CoV or 2019‐nCoV can both be used as vaccines to induce the production of cross‐reactive or cross‐neutralizing antibodies for preventing viruses from entering their target cells expressing its receptor ACE2. 10 Similarly, the S protein can also be used as a target for the creation of a COVID‐19 vaccine. Such vaccines can be based on the 2019‐nCoV full‐length S protein and its RBD; moreover, vaccines could be based on DNA, viral vectors and subunits. 11 A candidate messenger RNA vaccine stabilized the coronavirus S proteins by substituting two prolines at the top of heptad repeat 1, thereby preventing structural rearrangements of the fusion (S2) subunit, and successfully induced anti 2019‐nCoV immune responses in all participants. 12
Salvianolic acid A (SAA), salvianolic acid B (SAB), and salvianolic acid C (SAC) are phenolic carboxylic acids obtained from the aqueous extracts of Salvia miltiorrhiza. SAA, SAB, and SAC, which are major bioactive constituents of S. miltiorrhiza have been reported to have anticancer, anti‐inflammatory, and cardioprotective effects. 13 , 14 , 15 , 16 S. miltiorrhiza exhibits inhibitory effects against hepatitis B, hepatitis C, and SARS‐CoV viruses. 17 , 18 , 19 Although no drug has been confirmed to be effective in inhibiting 2019‐nCoV, treatment of COVID‐19 symptoms is still remarkable in clinic. 20 , 21 This is partly due to traditional Chinese medicine (TCM). 22 , 23 Salvia miltiorrhiza is one of the five main components of Xuebijing (XBJ) which is a five‐herb combined TCM, and other four components are Carthamus tinctorius, radix paeoniae rubra, Ligusticum wallichii, and Angelica sinensis. XBJ is widely used in China due to its anti‐inflammatory and anti‐endotoxin effects. A clinical study has shown that XBJ significantly improves the pneumonia severity index of patients with severe community‐acquired pneumonia. 24 Based on the clinical evidence for the benefits of XBJ in sepsis, bacterial pneumonia, and acute respiratory distress syndrome (ARDS), which are three common complications associated with COVID‐19, China's National Health Council has recommended i.v. XBJ as one of the treatment options in the management of COVID‐19 patients. 25 , 26 However, the efficacy and mechanism of SAA, SAB, and SAC in the treatment of COVID‐19 have not been established yet.
In this study, we have verified the inhibitory effects of SAA, SAB, and SAC on 2019‐nCoV spike pseudovirus infectivity in vitro and found that they can bind to the RBD of 2019‐nCoV spike pseudovirus, as well as to its receptor ACE2, to inhibit the entry of 2019‐nCoV spike pseudovirus into ACE2 high‐expressing HEK293T (ACE2h) cells.
2. MATERIALS AND METHODS
2.1. Materials and reagents
SAA and SAC were purchased from Herbest Pharmaceutical Co., Ltd. SAB was purchased from Ambrosia Pharmaceutical Co., Ltd. Chloroquine (CQ), purity of 98%, was from Macklin. Dulbecco's modification of Eagle's medium (DMEM) with high glucose (Cat. No.: SH30022.01), and fetal bovine serum (Cat. No.: 16140071) were from HyClone. Penicillin–streptomycin solution was obtained from Xi'an Hat Biotechnology Co., Ltd. The Annexin V‐FITC/PI Apoptosis Detection Kit (Cat. No.: A005‐3) and Cell Counting Kit were purchased from 7Sea Pharmatech Co., Ltd., the 2019‐nCoV spike pseudovirus (Cat: PSV001) was purchased from Sino Biological. The VSV‐G pseudovirus (Cat: GM‐2439LV18) was purchased from Genomeditech.
2.2. Cell culture
ACE2h cells were constructed by Genomeditech. ACE2h cells were maintained in DMEM high glucose medium containing 10% FBS, 1% penicillin‐streptomycin, 0.75 μg/ml puromycin and cultured at 37°C in a 5% CO2 incubator.
2.3. Cytotoxicity assay
Cell viability was evaluated according to the manufacturer's instructions. Briefly, ACE2h cells were seeded in 96‐well plates at a density of 5 × 103 cells per well and treated with different concentrations of SAA, SAB, and SAC (0, 25, 50, 100, 200, and 400 μM) for 24 h. Then, 10 μl of Cell Counting Kit solution was added to each well, followed by incubation for 2 h. Relative cell viability was assessed by measuring the absorbance at 450 nm using a microplate reader (Bio‐Rad). The survival rate of ACE2h cells was calculated using the following formula:
2.4. Apoptosis assay
ACE2h cells were seeded in a six‐well plate and treated with different concentrations of SAA, SAB, and SAC (0, 25, and 50 μM) for 24 h. Then, the cells were collected, washed with PBS and resuspended in 400 μl of 1 × binding buffer. Annexin V‐FITC (5 μl) was added to the cells and incubated 26°C in the dark for 15 min. Propidium iodide (PI) (10 μl) was added to the cells and incubated in an ice bath for 5 min. Detection was performed within 30 min. The excitation wavelength of the flow cytometer (Accuri C6 Plus; BD Biosciences) was 488 nm, and the emission wavelength was 530 nm to detect FITC, while PI was detected at 575 nm. Normal cells exhibited a low fluorescence intensity, while apoptotic cells exhibited a strong green fluorescence intensity. Necrotic cells exhibited double staining with green and red fluorescence.
2.5. Docking studies
Molecular docking studies were performed using SYBYL‐X 2.0 version. 6M0J (protein data bank [PDB] ID: 6M0J) is a crystal structure of SARS‐CoV‐2 spike receptor‐binding domain bound with human ACE2 protein. 6M0J has been published in the PDB. Subsequently, the compounds were screened based on the search space of the S‐RBD binding sites of human ACE2 (saved from 6M0J) using SYBYL‐X 2.0 version. Water molecules were removed and hydrogen was added. Tripos force field and Pullman charge were applied to minimize. SAA, SAB, and SAC were depicted by the Sybyl/Sketch module (Tripos Inc.), optimized by Powell's method with the Tripos force field with convergence criterion set at 0.005 kcal/(Å mol), and assigned using the Gasteiger–Hückel method.
2.6. Surface plasmon resonance
To assess the surface plasmon resonance (SPR), the ACE2 protein with a 6‐His tag (30 μg/ml) or SARS‐CoV‐2 spike S1 protein was fixed on a carboxyl sensor chip (Nicoya) via capture‐coupling. Then, 12.5, 25, 50, and 100 μM of SAA, SAB, and SAC were sequentially injected into the chamber, dissolved in a running buffer (PBS). The interaction of ACE2 with the fixed small molecules was observed using Open SPRTM (Nicoya Lifesciences) at 25°C. The binding and disassociation times were both 250 s and the flow rate was 20 μl/s, the chip was regenerated with hydrochloric acid (pH 2.0). A one‐to‐one diffusion‐corrected model was fitted to the wavelength shifts corresponding to the varied drug concentrations. The data were retrieved and analyzed using TraceDrawer.
2.7. Detection of 2019‐nCoV spike pseudovirus entry into ACE2h cells
White 96‐well plates were seeded with a solution of 5 × 104 of ACE2h cells in 100 μl of DMEM per well. The cells were cultured at a 37°C incubator containing 5% CO2; subsequently, the culture medium was removed and replaced with 100 μl of a medium containing the corresponding dose of the test substances, which was followed by incubation for 2 h. Then, 5 μl of 2019‐nCoV spike pseudovirus (PSC001; Sino Biological) was added. This was incubated in a 37°C incubator containing 5% CO2. After 6–8 h, the culture medium containing the virus was removed and replaced by 200 μl of fresh DMEM, and incubated continuously at 37℃ for 48 h; the culture medium was aspirated and 20 μl of cell lysate was added from the Luciferase Assay System (E1500; Promega) was added to each well. Then 100 μl of luminescence solution was added to each well. Chemiluminescence was detected at 560 nm using a microplate reader, and the exposure time was 1 s.
2.8. Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM) and were statistically analyzed using analysis of variance. Two‐tailed tests were used for comparisons between two groups, and differences were considered statistically significant at p < .05.
3. RESULTS
3.1. Cytotoxicity of SAA, SAB, and SAC in ACE2h cells
The effects of SAA, SAB, and SAC on ACE2h cell viability were determined using a Cell Counting Kit assay, by evaluating their cytotoxic effects. Cytotoxic screening of SAA, SAB, and SAC was performed at different concentrations in the range of 25–400 μM. SAB and SAC showed low cytotoxicity towards ACE2h cells even at a concentration as high as 400 μM. SAA exhibited an inhibitory effect on cell viability at concentrations above 200 μM (Figure 1B). It can be concluded that at a given concentration, SAA, SAB, and SAC showed low toxicity on ACE2h cells at different time points (Figure 1C). Figure 2 shows that within 24 h, the concentrations of all three salvianolic acids had no significant effect on apoptosis in the later experiments.
Figure 1.
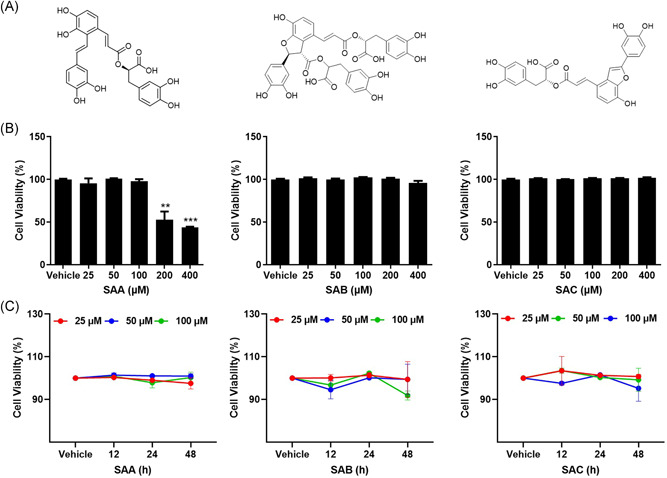
Effect of cell viability of ACE2h cells by SAA, SAB, and SAC. (A) Structural formulae of SAA, SAB, and SAC; (B) viability of ACE2h cells treated with SAA, SAB, and SAC for 24 h; (C) the toxicity of SAA, SAB, and SAC on ACE2h cells at different time points. The experiments were repeat three times. Data are presented as mean ± SD. *p < .05, **p < .01, ***p < .001 compared with vehicle. ACE, angiotensin‐converting enzyme 2; SA, salvianolic acid; SD, standard deviation
Figure 2.
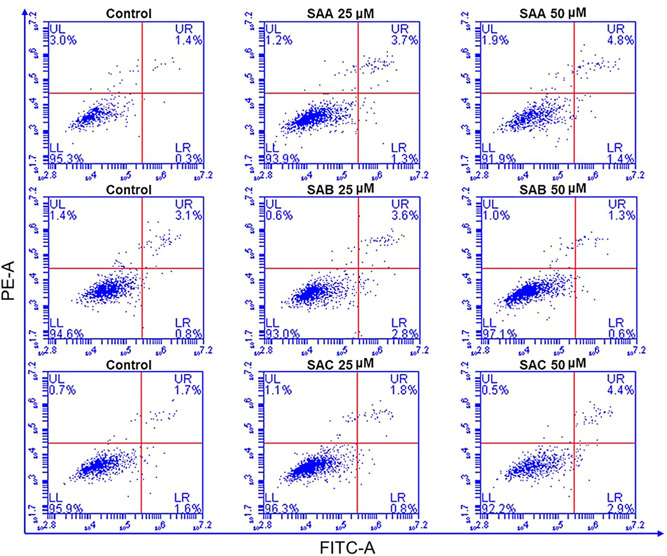
The apoptosis of ACE2h cells treated with SAA, SAB, and SAC for 24 h. The experiments were repeated three times. ACE, angiotensin‐converting enzyme 2; SA, salvianolic acid
3.2. Binding capacities of SAA, SAB, and SAC to the RBD of 2019‐nCoV
Existing studies have proved that the 2019‐nCoV infects its host cells through binding to ACE2 followed by cleavage of the S protein of 2019‐nCoV by human TMPRSS2. 6 This binding triggers a cascade of events that lead to the fusion of the cell and viral membranes, allowing for entry into the host cell. 9 Therefore, we analyzed the binding capacities of SAA, SAB, and SAC to the RBD of 2019‐nCoV using molecular docking. Figure 3A showed that SAA binds to GLN498, which is an active site on the RBD of 2019‐nCoV via the formation of a 2.12 Å hydrogen bond. SAC binds to ARG403, GLU406, GLU484, and PHE490, which are all invalid sites of the RBD of 2019‐nCoV (Table 1). Notably, we found that SAB showed a stronger binding ability to the RBD through the formation of more hydrogen bonds. SAB binds to six amino acids of the RBD of 2019‐nCoV. Among them, SAB binds to the active amino acid TYR453 (via the formation of 1.91 Å and 2.25 Å hydrogen bonds), ASN501 (via the formation of a 1.91 Å hydrogen bonds) and THR500 (via the formation of 2.43 Å, 1.78 Å, 2.21 Å, 2.41 Å, and 2.37 Å hydrogen bonds) (Table 1). We used SPR to further assess the binding abilities of the three salvianolic acids to the RBD of 2019‐nCoV. The binding constant (K D ) between SAA, SAB, SAC, and S protein of 2019‐nCoV was (3.82 ± 0.43)e−6 M, (5.15 ± 0.64)e−7 M, and (2.19 ± 0.14)e−6 M, respectively (Figure 3B). These results further confirmed that SAB has a greater affinity for the RBD of 2019‐nCoV S protein than SAA and SAC.
Figure 3.
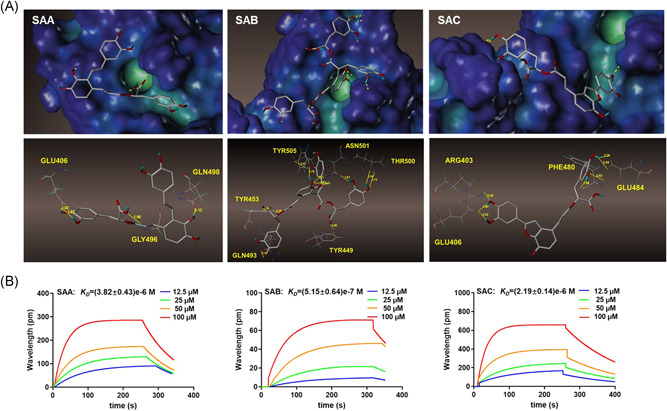
The binding character of SAA, SAB, and SAC on the S protein of 2019‐nCoV. (A) The molecular docking of SAA, SAB, and SAC with S protein of 2019‐nCoV; (B) SPR analysis of SAA, SAB, and SAC with S protein of 2019‐nCoV. nCoV, new coronavirus; SA, salvianolic acid; SPR, surface plasmon resonance
Table 1.
Amino acid residues that form hydrogen bonds between the three salvianolic acids and ACE2 or RBD
| ACE2 | RBD | |
|---|---|---|
| SAA | TYR83, GLU35, LYS31, HIS34a | GLU406, GLN498,a GLY496 |
| SAB | ASN330, GLU329, ARG357,a LYS353,a ASP38, TYR41,a GLN42a | TYR453,a GLN493, TYR449, TYR505, ASN501,a THR500a |
| SAC | ARG393, LYS353,a LYS31 | ARG403, GLU406, GLU484, PHE490 |
Abbreviations: ACE, angiotensin‐converting enzyme 2; RBD, receptor‐binding domain.
Amino residues are the active sites of ACE2 binding to RBD during virus invasion.
3.3. Binding abilities of SAA, SAB, and SAC to ACE2
Based on the results of the study on the structure of ACE2, 9 we evaluated the binding abilities of SAA, SAB, and SAC to ACE2 using molecular docking and focused on whether SAA, SAB, and SAC could bind to ACE2. We found that SAA, SAB, and SAC all have good binding abilities, with respect to ACE2 protein (Figure 4A). Figure 4A shows that SAA binds to HIS34, which is an active site on the ACE2, by forming a 2.09 Å hydrogen bond and three invalid amino acids, GLU35, LYS31, TYR83. SAC bind to LYS353, which is an active site on the ACE2 by forming a 2.02 Å hydrogen bond and two invalid amino acids, ARG393, and LYS31 (Table 1). Notably, SAB showed a stronger binding ability to ACE2 via the formation of much more hydrogen bonds. SAB binds to the active amino acids, ARG357, LYS353, TYR41, GLN42, and three invalid amino acids, ASN330, GLU329, ASP393 (Table 1). We used SPR to further assess the binding abilities of the three salvianolic acids to ACE2. The K D between SAA, SAB, and SAC and ACE2 protein was (4.08 ± 0.61)e−7 M, (2.95 ± 0.78)e−7 M, and (7.32 ± 0.42)e−7 M, respectively (Figure 4B). These results further confirmed that SAB has a greater affinity for ACE2 than SAA and SAC.
Figure 4.
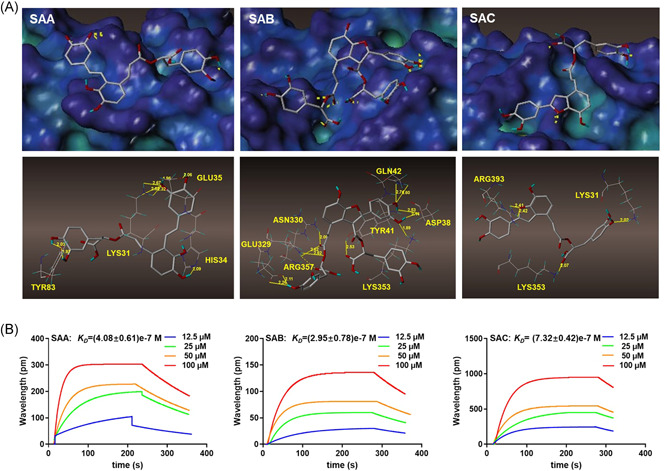
The binding character of SAA, SAB, and SAC on ACE2. (A) The molecular docking of SAA, SAB, and SAC with ACE2 protein; (B) SPR analysis of SAA, SAB and SAC with ACE2 protein. ACE, angiotensin‐converting enzyme 2; SA, salvianolic acid
3.4. Effects of SAA, SAB, and SAC on the entry of 2019‐nCoV spike pseudovirus into ACE2h cells
The pseudovirus system is a classic model to study the entry process of enveloped viruses, as well as to assess the activity of antiviral agents targeting the virus entry stage. Here, we developed a Pseudovirus system using SARS‐CoV‐2 S protein to study the virus entry and tested SAA, SAB, and SAC on this assay. There are reports that chloroquine (CQ) can effectively treat COVID, and studies have shown that it can effectively inhibit the entry of the 2019‐nCoV spike pseudovirus into ACE2h cells. 27 Therefore, CQ was selected as the positive control for the experiment. The luciferase luminescence value of ACE2h cells infected with 2019‐nCoV spike pseudovirus was defined as 1. Under the treatment with 20 μM SAA, SAB, and SAC, the 2019‐nCoV spike pseudovirus entry ratio was reduced to 0.72 ± 0.09, 0.43 ± 0.10, and 0.66 ± 0.08, respectively (Figure 5). The inhibitory effects of SAB on the entry of 2019‐nCoV spike pseudovirus into ACE2h cells were greater than those of SAA and SAC. As a result, SAA, SAB, and SAC were determined to inhibit the entry of 2019‐nCoV spike pseudovirus with EC50 of 11.31, 6.22, and 10.14 μM on ACE2h cells, respectively (Figure 6), while no inhibitory activities were observed on vesicular stomatitis virus glycoprotein (VSV‐G) pseudovirus under the treatment of SAA, SAB, and SAC (Figure S1).
Figure 5.
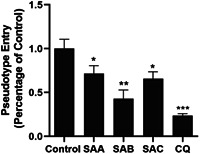
The different effects of 20 μM SAA, SAB, and SAC on the entry of 2019‐nCoV spike pseudovirus into ACE2h cells. The experiments were repeat three times. Data are presented as mean ± SD. *p < .05, **p < .01, ***p < .001 compared with control. ACE, angiotensin‐converting enzyme 2; nCoV, new coronavirus; SA, salvianolic acid; SD, standard deviation
Figure 6.
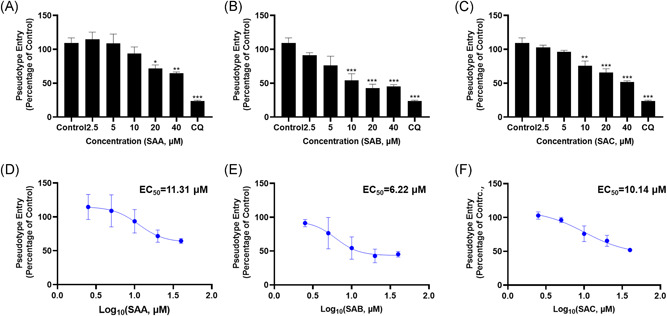
SAA, SAB, and SAC inhibited the entry of SARS‐CoV‐2 pseudovirus on ACE2h cells. The experiments were repeat three times. Data are presented as mean ± SD. *p < .05, **p < .01, ***p < .001 compared with control. ACE, angiotensin‐converting enzyme 2; SA, salvianolic acid; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation
4. DISCUSSION
COVID‐19 has been affecting the health of individuals globally, and there are currently no specific drugs against the 2019‐nCoV virus infection. In this study, we found that SAA, SAB, and SAC which are the main active ingredients of S. miltiorrhiza, interacted with both RBD of 2019‐nCoV and its receptor ACE2 and further inhibited the entry of 2019‐nCoV spike pseudovirus into ACE2h cells.
Earlier studies in other cells such as HEK293/ETAR cell line, human mesenchymal stem cells and neuronal cells revealed that the inhibitory effect of SAA, SAB, and SAC had a minor effect on cells viability. 28 , 29 , 30 In this study, at concentrations greater than 200 μM, only SAA showed ACE2h cytotoxicity and decreases cell viability. Moreover, the concentrations we chose in our experiment were below the toxic dose. To further evaluate the effects of SAA, SAB, and SAC on ACE2h cell apoptosis, SAA, SAB, or SAC has no effect on ACE2h cell apoptosis at 50 μM with the apoptosis rate of less than 10%.
The binding of 2019‐nCoV with ACE2 is mediated mainly through polar interactions. 9 As with SARS‐CoV, S1 subunits of 2019‐nCoV contains the RBD, which directly binds to the peptidase domain (PD) of ACE2; whereas S2 is responsible for membrane fusion. 31 , 32 An extended loop region of the RBD spans the arch‐shaped α1 helix of ACE2‐PD like a bridge. 9 The contact can be divided into three clusters. The two ends of the bridge interact with the N and C termini of the α1 helix, and two polar residues are involved in strengthening the interaction in the middle segment of α1. 9 From a structural perspective, SAA, SAB, and SAC can bind to active sites on RBD or ACE2‐PD to block their polar interactions. SAA can bind to GLN498 on the RBD, as well as HIS34 in the middle segment of α1 helix of ACE2‐PD. SAC can only bind to LYS353 for blocking the interaction with C termini of the α1 helix. Interestingly, SAB can bind to almost all active sites that play important roles in the interactions of RBD and the N termini of the α1 helix of ACE2‐PD and generates more hydrophilic groups. Moreover, SAB can bind to TYR453 in the middle segment of α1. Therefore, we concluded that SAA, SAB and SAC could bind strongly to 2019‐nCoV‐RBD, as well as to its receptor ACE2. SAB could display a stronger binding ability to 2019‐nCoV and ACE2 than SAA and SAC due to their structural differences. Our SPR further confirmed that the salvianolic acids showed strong binding to 2019‐nCoV‐RBD and ACE2. SAB can bind to 2019‐nCoV‐RBD or its receptor ACE2, resulting in a stronger binding effect than that of SAA and SAC.
A key step in the viral infection process is the entry of viruses into cells. 27 Considering the safety factors involved, the throughput and accessibility of live‐virus neutralization assays on 2019‐nCoV are limited by the fact that the virus is a biosafety‐level‐3 agent that must be handled in specialized facilities. 33 Therefore, we used the 2019‐nCoV spike pseudovirus for this study. Pseudovirus refers to a retrovirus that can integrate the membrane glycoproteins of a different kind of virus to form an external viral membrane, while the genome retains the genomic characteristics of the retrovirus itself. 34 The pseudovirus can only infect cells once and is extremely safe. Although the pseudovirus cannot portray all the characteristics of the live‐2019‐nCoV, it can be used to simulate the process of viruses entry into cells and evaluate the antiviral activities of compounds in vitro. 35 In this study, we used the 2019‐nCoV spike pseudovirus to infect ACE2h cells, as an infection model, to evaluate the antiviral activities of SAA, SAB, and SAC. The results show that SAA, SAB, and SAC can effectively inhibit the entry of viruses into cells. In addition, the antiviral properties of SAB are greater than that those of SAA and SAC, indicating that SAB may inhibit the binding of the 2019‐nCoV spike pseudovirus to ACE2. Recent studies on SAC confirmed its inhibitory effects on live‐2019‐nCoV. 36 According to our research, we speculate that SAB may show a better inhibition effect, with respect to the entry process of the 2019‐nCoV pseudovirus into ACE2h cells. Nevertheless, whether SAC can elicit greater inhibitory effects than SAB in other key steps in live‐2019‐nCoV infection, such as infection, replication, and release, resulting in better antiviral effects, is yet to be determined.
In conclusion, our study showed that SAA, SAB, and SAC inhibit 2019‐nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2, suppressing the entry of 2019‐nCoV pseudovirus into ACE2h cells. Among these compounds, SAB showed the greatest binding affinity and anti‐2019‐nCoV pseudovirus effect; this provides new insights into the use of traditional Chinese medicine for the treatment and control of COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Writing: Shiling Hu, Nan Wang, and Langchong He. Methodology: Shiling Hu, Jue Wang, and Yongjing Zhang. Data analysis: Cheng Wang and Haoyun Bai.
PEER REVIEW
The peer review history for this article is available at http://publons.com/publon/10.1002/jmv.26874
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was co‐funded by the National Natural Science Foundation of China (Grant number: 81930096) and the China Postdoctoral Science Foundation (Grant number: 2019M653672).
Hu S, Wang J, Zhang Y, et al. Three salvianolic acids inhibit 2019‐nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2. J Med Virol. 2021;93:3143–3151. 10.1002/jmv.26874
Contributor Information
Nan Wang, Email: wangnan2014@xjtu.edu.cn.
Langchong He, Email: helc@mail.xjtu.edu.cn.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Ghafouri‐Fard S, Noroozi R, Omrani MD, et al. Angiotensin converting enzyme: a review on expression profile and its association with human disorders with special focus on SARS‐CoV‐2 infection. Vascul Pharmacol. 2020;130:106680. 10.1016/j.vph.2020.106680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363‐374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025‐1031. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lukassen S, Chua RL, Trefzer T, et al. SARS‐CoV‐2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215‐220. 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- 8. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084‐H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444‐1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tai W, He L, Zhang X, et al. Characterization of the receptor‐binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613‐620. 10.1038/s41423-020-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS‐CoV‐‐a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226‐236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2—preliminary report. N Engl J Med. 2020;383:1920‐1931. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin T, Rasul A, Sarfraz A, et al. Salvianolic acid A & B: potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int J Biol Sci. 2019;15(10):2256‐2264. 10.7150/ijbs.37467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng S, Cong H, Ji L. Salvianolic acid A exhibits anti‐inflammatory and antiarthritic effects via inhibiting NF‐kappaB and p38/MAPK pathways. Drug Des Devel Ther. 2020;14:1771‐1778. 10.2147/DDDT.S235857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen B, Huang C, Zhang Y, et al. Salvia bowleyana Dunn root is a novel source of salvianolic acid B and displays antitumor effects against gastric cancer cells. Oncol Lett. 2020;20(1):817‐827. 10.3892/ol.2020.11611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Li L, Fang G. Salvianolic acid A attenuates cerebral ischemia/reperfusion injury induced rat brain damage, inflammation and apoptosis by regulating miR‐499a/DDK1. Am J Transl Res. 2020;12(7):3288‐3301. [PMC free article] [PubMed] [Google Scholar]
- 17. Cui XY, Wang YL, Kokudo N, Fang DZ, Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends. 2010;4(2):39‐47. [PubMed] [Google Scholar]
- 18. Tsai FJ, Cheng CF, Chen CJ, et al. Effects of Chinese herbal medicine therapy on survival and hepatic outcomes in patients with hepatitis C virus infection in Taiwan. Phytomedicine. 2019;57:30‐38. 10.1016/j.phymed.2018.09.237 [DOI] [PubMed] [Google Scholar]
- 19. Park JY, Kim YM, Ryu YB, Lee WS. Tanshinones as selective and slow‐binding inhibitors for Sars‐Cov cysteine proteases. Pharm Biol. 2012;50(11):1361‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciliberto G, Mancini R, Paggi MG. Drug repurposing against COVID‐19: focus on anticancer agents. J Exp Clin Cancer Res. 2020;39(1):86. 10.1186/s13046-020-01590-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boussageon R, Pouchain D, Le Roux G. Hydroxychloroquine and azithromycin as a treatment of Covid‐19: results of an open label non‐randomized clinical trial. Exercer. 2020;162:170‐171. 10.1016/j.ijantimicag.2020.105949 [DOI] [Google Scholar]
- 22. Wang WY, Xie Y, Zhou H, Liu L. Contribution of traditional Chinese medicine to the treatment of COVID‐19. Phytomedicine. 2020:153279. 10.1016/j.phymed.2020.153279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du HZ, Hou XY, Miao YH, Huang BS, Liu DH. Traditional Chinese medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin J Nat Med. 2020;18(3):206‐210. 10.3724/Sp.J.1009.2019.000000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song Y, Yao C, Yao Y, et al. Xuebijing injection versus placebo for critically Ill patients with severe community‐acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47(9):e735‐e743. 10.1097/CCM.0000000000003842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang H, Ji L, Song S, et al. Identification of the major constituents in Xuebijing injection by HPLC‐ESI‐MS. Phytochem Anal. 2011;22(4):330‐338. 10.1002/pca.1284 [DOI] [PubMed] [Google Scholar]
- 26. Zuo L, Sun Z, Hu Y, et al. Rapid determination of 30 bioactive constituents in Xuebijing injection using ultra high performance liquid chromatography‐high resolution hybrid quadrupole‐orbitrap mass spectrometry coupled with principal component analysis. J Pharm Biomed Anal. 2017;137:220‐228. 10.1016/j.jpba.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 27. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Q, Wang S, Yu Y, et al. Salvianolic acid A, as a novel ETA receptor antagonist, shows inhibitory effects on tumor in vitro. Int J Mol Sci. 2016;17(8):1244. 10.3390/ijms17081244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu D, Xu L, Zhou C, et al. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol. 2014;51:1‐9. 10.1016/j.biocel.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 30. He Y, Jia K, Li L, et al. Salvianolic acid B attenuates mitochondrial stress against Abeta toxicity in primary cultured mouse neurons. Biochem Biophys Res Commun. 2018;498(4):1066‐1072. 10.1016/j.bbrc.2018.03.119 [DOI] [PubMed] [Google Scholar]
- 31. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309(5742):1864‐1868. [DOI] [PubMed] [Google Scholar]
- 32. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA. 2009;106(14):5871‐5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Frontiers of medicine. 2020;14(2):185‐192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang N, Han S, Liu R, et al. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019‐nCoV Spike pseudotyped virus. Phytomedicine. 2020;79:153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Delvecchio R, Higa L, Pezzuto P, et al. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8(12):322. 10.3390/v8120322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang C, Pan X, Xu X, et al. Salvianolic acid C potently inhibits SARS‐CoV‐2 infection by blocking the formation of six‐helix bundle core of spike protein. Signal Transduct Target Ther. 2020;5(1):220. 10.1038/s41392-020-00325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data available on request from the authors.


