Dear Editor, To date, the COVID‐19 pandemic has affected over 85 million persons worldwide, with 14% of cases severe and 5% critical, and a general population case‐fatality rate of 1%. Age is the main risk factor for severe disease and death,1 making residents of long‐term care facilities (LTCFs) particularly vulnerable.2 Therapeutic agents for COVID‐19 remain sparse, but ivermectin, an antiparasitic, has shown anti‐SARS‐CoV‐2 activity in vitro.3 Moxidectin, another macrocyclic lactone, with a longer plasma half‐life, could also be considered.4
On 6 March 2020, a 66‐year‐old woman (Resident 1) with numerous comorbidities, from LTCF‐A (Seine‐et‐Marne county, in suburban Paris, France) was referred to our dermatology department with profuse scabies. She was treated in our randomized controlled trial (NCT02841215) with ivermectin 400 or 200 µg kg−1 (exact dose double‐blinded) on days 0, 7 and 14. LTCF‐A identified three more residents with scabies, residing on different floors, and declared an outbreak. They were treated accordingly, and all remaining LTCF‐A residents and staff (n = 117) were also treated simultaneously with ivermectin (200 µg kg−1, days 0–7, starting 10 March 2020) (Figure 1).
Figure 1.
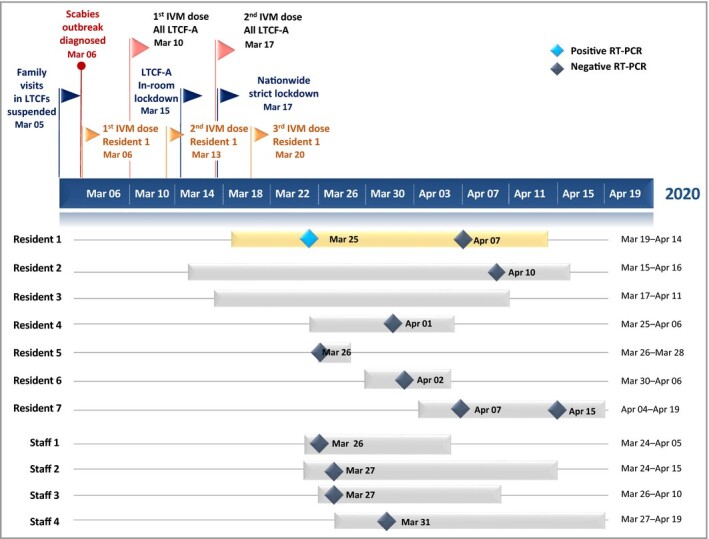
Concomitant COVID‐19 and ivermectin‐treated scabies outbreak in a long‐term care facility. The navy blue timeline represents COVID‐19‐dictated closure coinciding with onset of a scabies outbreak in long‐term care facility A (LTCF‐A), and the oral ivermectin (IVM) treatments for the scabies‐infested patients (n = 4) and their contacts (n = 117), for a total of 121 IVM‐treated individuals, according to NCT02841215 and current therapeutic recommendations on scabies. The bars below the timeline represent the different individuals with confirmed or suspected COVID‐19 (LTCF‐A residents and staff). Real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) (RdRp gene, CT 36 and N gene, CT 34) detected Resident 1’s COVID‐19 (yellow bar).
All confirmed or suspected cases of COVID‐19 among LTCF‐A’s residents and staff from 5 March to 15 May 2020 were identified. LTCF‐A residents’ demographics (age and sex) and COVID‐19 clinical outcomes (recovery, hospitalization or death) were recorded. During the same period (5 April to 1 May 2020; no prior data available), all confirmed COVID‐19 cases and deaths in all other LCTFs in Seine‐et‐Marne were systematically declared in the French national online database (https://signalement.social‐sante.gouv.fr) and all data were gathered by the Agence Régionale de Santé–Ile‐de‐France (https://www.iledefrance.ars.sante.fr).
We selected all county‐wide LTCFs comparable with LTCF‐A in terms of fees and size (50–90 residents). All remaining LTCFs provided aggregated demographic data (age and sex). Each retained LTCF – except LTCF‐A – was considered a cluster. R software (v6.1.2; R Foundation, Vienna, Austria) was used to estimate COVID‐19 infection and mortality rates, and their distributions. In parallel, an in vitro virological study measured the anti‐SARS‐CoV‐2 activity of ivermectin and moxidectin on VeroE6‐monkey kidney cells at increasing concentrations (range 0·05–10 µmol L−1) by RNA quantification and immunofluorescence (with cell viability controlled at each step). We also performed a time‐of‐drug‐addition assay.
Between 5 March and 15 May 2020, 69 LTCF‐A residents (median age 90 years, interquartile range 84–94; 78% female) and 52 staff members received ivermectin (Figure 1). Eleven persons presented confirmed or suspected COVID‐19 (1·4% declared in the ARS online database), with the first symptoms noticed on 11 March 2020 (Resident 1 on 19 March 2020). One resident (Resident 1) had a SARS‐CoV‐2‐positive reverse‐transcriptase polymerase chain reaction. No hospitalizations and no deaths were noted. Forty‐five ‘matched’ county‐wide LTCFs were included as a reference sample (out of 177), housing 3062 residents (median age 86 years, interquartile range 87–89; 77·3% female). Among them, a mean of 22·6% (95% confidence interval 16·3–28·9) acquired declared COVID‐19, with a lethality of 4·9% (95% confidence interval 3·2–6·5).
The virological study confirmed important in vitro antiviral activity, with EC50 values of 0·14 ± 0·02 µmol L−1 and 0·48 ± 0·08 µmol L−1 for ivermectin and moxidectin, respectively. The maximum inhibitions at 5 µmol L−1 were 55 000 and 19 000‐fold, respectively, without affecting cell viability. The study also showed dose‐dependently limited numbers of SARS‐CoV‐2‐infected cells, and complete inhibition of SARS‐CoV‐2 infection at 2·5 µmol L−1. When ivermectin and moxidectin were added 3 h after infection (i.e. early during SARS‐CoV‐2 infection), no antiviral effect was seen (data not shown).
To control scabies, the entire LTCF‐A population was given ivermectin, while at the same time a COVID‐19 outbreak was declared. No ivermectin‐exposed LTCF‐A resident developed severe COVID‐19 or died, while residents from control LTCFs showed higher COVID‐19 rates. Usually, once COVID‐19 enters an LTCF – in any healthcare system – its rapid dissemination5 is associated with a high risk of death. Ivermectin is an antiparasitic drug that is used to treat neglected tropical diseases such as onchocerciasis, helminthiases and scabies, and is evaluated at high doses for malaria control. It could have a protective role in COVID‐19 within a therapeutic margin,6 as supported by our optimized virological study. Other populations might have already benefited from ivermectin, as its use was associated with lower in‐hospital mortality in a multihospital retrospective cohort study of 280 North American patients [ivermectin, n = 173 (15%) vs. no ivermectin, n = 107 (25·2%), odds ratio 0·52, 95% confidence interval 0·29–0·96; P = 0·03].7 There are both epidemiological (i.e. the ecological nature of the data with probability of unmeasured confounding) and virological limitations of the available data, and difficulties in extrapolating in vitro antiviral effects against different coronaviruses to clinical efficacy. However, the plausibility is sufficient to carry out further studies to elucidate whether ivermectin (and moxidectin) is or is not an appropriate candidate for the prevention of COVID‐19.
Acknowledgments
We would like to gratefully acknowledge all of the residents, healthcare workers and administrative staff of LTCF‐A and their directors for agreeing to participate in this report, and providing their technical support. We acknowledge Margaut Petignier, ARS Ile‐de‐France, for assistance in generating COVID‐19 and mortality rate data in Seine‐et‐Marne county, and all of the certified nurse assistants and administrative staff of the 45 LRCFs that agreed to share their demographic information. We thank Saskia Ingen‐Housz‐Oro, Françoise Foulet, Audrey Colin and the nursing staff of the Dermatology Department, Henri‐Mondor Hospital, Créteil, for their devoted patient care; Hayat Medjenah, Elie Guichard, Laetitia Grégoire, the AGEPS pharmacists and the administrative staff of the Unité de Recherche Clinique, Henri‐Mondor Hospital, Créteil, for NCT02841215 RCT support; Françoise Botterel and Jacques Guillot for their assistance supervising preliminary laboratory work; Selim Aractingi, Jean‐Philippe Derenne, Anaïs Farcet, Bruno Housset, Pascal del Giudice, Fatimata Ly and Solène Makdessi for discussions and thoughtful advice; Anne‐Claude Crémieux for the specific COVID‐19 literature weekly review of APHP hospitals; and Janet Jacobson for editorial assistance.
Author Contribution
Charlotte Bernigaud: Conceptualization (equal); Data curation (lead); Formal analysis (lead); Funding acquisition (supporting); Investigation (lead); Methodology (equal); Project administration (lead); Software (supporting); Validation (equal); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Didier GUILLEMOT: Conceptualization (supporting); Data curation (supporting); Formal analysis (equal); Investigation (supporting); Methodology (equal); Software (lead); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Hakim Ahmed‐Belkacem: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Resources (supporting); Software (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting). Lamiae Grimaldi‐Bensouda: Data curation (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting). Anne Lespine: Data curation (supporting); Formal analysis (supporting); Methodology (supporting); Validation (supporting); Writing‐original draft (supporting). Claude Chesnot: Data curation (supporting); Formal analysis (supporting); Investigation (supporting); Project administration (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting). giao Do: Data curation (supporting); Formal analysis (supporting); Funding acquisition (equal); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Writing‐original draft (supporting). Bruno Giraudeau: Formal analysis (supporting); Investigation (supporting); Methodology (equal); Supervision (equal); Validation (supporting); Writing‐original draft (supporting). Slim Fourati: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐original draft (supporting). Olivier Chosidow: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Supervision (lead); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
References
- Williamson E, Walker AJ, Bhaskaran KJ. et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael TM, Currie DW, Clark S. et al. Epidemiology of COVID‐19 in a long‐term care facility in King County, Washington. N Engl J Med 2020; 382:2005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L, Druce JD, Catton MG. et al. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res 2020; 178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo CA, Furtek CI, Porras AG. et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002; 42:1122–33. [DOI] [PubMed] [Google Scholar]
- Arons MM, Hatfield KM, Reddy SC. et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M, Rayner C, Noël F. et al. Ivermectin and COVID‐19: a report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antiviral Res 2020; 178:104805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepelowicz Rajter J, Sherman M, Fatteh N. et al. Use of ivermectin is associated with lower mortality in hospitalized patients with COVID‐19 (ICON study). Chest 2021; 159:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


