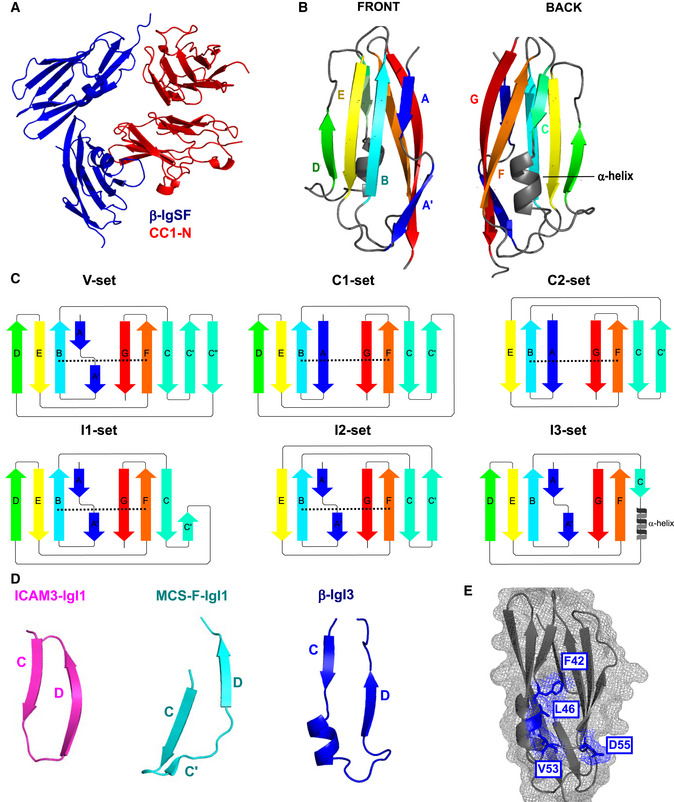
Illustration of the secondary structure organisation of the V‐set, C1‐set, C2‐set, I1‐set, I2‐set and I3‐set domains. Each secondary structure is composed of varying combinations of the β‐strands A, A′, B, C, C′, C′′, D, E, F, G. Disulphide bridges between the B strand and F strand are represented as a black dashed lines. The C‐set domains lack the A′ and C′′ strands and are subtyped into C1‐set and C2‐set based on presence and absence of the D strand, respectively. The I‐set domains lack C′′ strand and are subtyped into C1‐set and C2‐set based on presence and absence of the D strand, respectively. The IgSF fold from β protein has the I3‐set domain, characterised by absence of disulphide bridge, presence of the D strand, and presence of a truncated C strand followed by a 1.5‐turn α‐helix.