Abstract
Circadian rhythms are a pervasive property of mammalian cells, tissues and behaviour, ensuring physiological adaptation to solar time. Models of cellular timekeeping revolve around transcriptional feedback repression, whereby CLOCK and BMAL1 activate the expression of PERIOD (PER) and CRYPTOCHROME (CRY), which in turn repress CLOCK/BMAL1 activity. CRY proteins are therefore considered essential components of the cellular clock mechanism, supported by behavioural arrhythmicity of CRY‐deficient (CKO) mice under constant conditions. Challenging this interpretation, we find locomotor rhythms in adult CKO mice under specific environmental conditions and circadian rhythms in cellular PER2 levels when CRY is absent. CRY‐less oscillations are variable in their expression and have shorter periods than wild‐type controls. Importantly, we find classic circadian hallmarks such as temperature compensation and period determination by CK1δ/ε activity to be maintained. In the absence of CRY‐mediated feedback repression and rhythmic Per2 transcription, PER2 protein rhythms are sustained for several cycles, accompanied by circadian variation in protein stability. We suggest that, whereas circadian transcriptional feedback imparts robustness and functionality onto biological clocks, the core timekeeping mechanism is post‐translational.
Keywords: cellular clock, circadian rhythm, cryptochrome, daily timekeeping, robustness
Subject Categories: Signal Transduction
Circadian timekeeping in the absence of CRYPTOCHROME provides further support to the central role of post‐translational regulation in the mammalian circadian clock mechanism.
Introduction
The adaptive advantage conferred on organisms by anticipation of the 24‐h cycle of day and night has selected for the evolution of circadian clocks that, albeit in different molecular forms, are present throughout all kingdoms of life (Rosbash, 2009; Edgar et al, 2012). Circadian rhythms are robust, in that they are “capable of performing without failure under a wide range of conditions” (Merriam‐Webster Dictionary, 2020). The mechanism proposed to generate daily timekeeping in mammalian cells is a delayed transcriptional–translational feedback loop (TTFL) that consists of activating transcription factor complexes containing CLOCK and BMAL1 and repressive complexes, containing the BMAL1:CLOCK targets PERIOD and CRYPTOCHROME (reviewed in Dunlap, 1999; Reppert & Weaver, 2002; Takahashi, 2016). Various coupled, but non‐essential, auxiliary transcriptional feedback mechanisms are thought to fine‐tune the core TTFL and co‐ordinate cell‐type‐specific temporal organisation of gene expression programs; the best characterised being effected by the E‐box mediated rhythmic expression of REV‐ERBα/β, encoded by the Nr1d1/2 genes (Preitner et al, 2002; Ueda, 2007; Liu et al, 2008; Takahashi, 2016). These auxiliary loops are not considered sufficient to generate circadian rhythms in the absence of the core TTFL (Preitner et al, 2002; Liu et al, 2008).
CRY1 and CRY2 operate semi‐redundantly as the essential repressors of CLOCK/BMAL1 activity (Ye et al, 2014; Chiou et al, 2016), required for the nuclear import of PER proteins, and together are considered indispensable for circadian regulation of gene expression in vivo as well as in cells and tissues cultured ex vivo (Kume et al, 1999; Sato et al, 2006; Chiou et al, 2016; Ode et al, 2017). Certainly, mice homozygous null for Cry1 and Cry2 do not express circadian behavioural rest/activity cycles under standard experimental conditions (Thresher et al, 1998; Horst & Muijtjens, 1999; Vitaterna et al, 1999).
The hypothalamic suprachiasmatic nucleus (SCN) is a central locus for circadian co‐ordination of behaviour and physiology, and research over the last two decades has stressed the strong correlation between SCN timekeeping in vivo and its activity when cultured ex vivo (Welsh et al, 2010; Anand et al, 2013). We were therefore intrigued by the observation that roughly half of organotypic SCN slices prepared from homozygous Cry1 −/−,Cry2 −/− (CRY knockout; CKO) mouse neonates continue to exhibit ~ 20 h (short period) rhythms, observed using the genetically encoded PER2::LUC clock protein::luciferase fusion reporter (Maywood et al, 2011; Ono et al, 2013b), despite having previously been described as arrhythmic (Liu et al, 2007). Moreover, short period circadian rhythms of locomotor activity have previously been reported for CKO mice raised from birth under constant light (Ono et al, 2013a). As CKO SCN oscillations were only observed in cultured neonatal organotypic slices ex vivo, they were suggested to be a network‐level SCN‐specific rescue by the activity of neuronal circuits, that desynchronise during post‐natal development (Welsh et al, 2010; Ono et al, 2013b). In our view, however, these observations are difficult to reconcile with an essential requirement for CRY in the generation of circadian rhythms. Rather, they are more consistent with CRY making an important contribution to circadian rhythm stability and functional outputs, rather than to the timekeeping mechanism per se, as recently shown for the genes Bmal1 and Clock (Landgraf et al, 2016; Ray et al, 2020), which had both previously been thought indispensable for circadian timekeeping in individual cells (Bunger et al, 2000; DeBruyne et al, 2007). This is further supported by reports that constitutive expression of Cry1 in cells and SCN perturbs but does not abolish circadian oscillations (Fan et al, 2007; Chen et al, 2009; Nangle et al, 2014; Edwards et al, 2016).
Recent observations have further questioned the need for transcriptional feedback repression to enable cellular circadian timekeeping. For example, circadian protein translation is regulated by cytosolic BMAL1 through a transcription‐independent mechanism (Lipton et al, 2015), and isolated erythrocytes exhibit circadian rhythms despite lacking any DNA (O’Neill & Reddy, 2011; Cho et al, 2014). Moreover, circadian timekeeping in some species of eukaryotic alga and prokaryotic cyanobacteria can occur entirely post‐translationally (Sweeney & Haxo, 1961; Nakajima et al, 2005; Tomita et al, 2005; O’Neill et al, 2011). Whether non‐transcriptional clock mechanisms operate in other (nucleated) mammalian cells is unknown however, and hence their mechanism and relationship with TTFL‐mediated rhythms is an open question.
Here, we used cells and tissues from CRY‐deficient mice, widely accepted not to exhibit circadian transcriptional regulation (Kume et al, 1999; Ukai‐Tadenuma et al, 2011; Edwards et al, 2016) to test whether any timekeeping function remained from which we might begin to dissect the mechanism of the postulated transcription‐independent cytosolic oscillator or “cytoscillator” (Hastings et al, 2008).
Results
Cell‐autonomous circadian PER2::LUC rhythms in the absence of CRY proteins
Consistent with previous observations, we found no significant circadian organisation of locomotor activity in CRY‐deficient (CKO) mice following entrainment to 12 h:12 h light:dark (LD) cycles or in constant light (LL). Upon transition from constant light to constant darkness (DD) [described to be a stronger zeitgeber (Chen et al, 2008)] however, CKO mice expressed rhythmic bouts of consolidated locomotor activity with an average period of ~ 17 h and greater variance than WT controls (Figs 1A and B, and EV1A–C). In Fig 1, representative actograms are plotted as a function of endogenous tau (τ) to allow the periodic organisation of rest–activity cycles to be readily observed; 24‐h‐plotted actograms are shown in Figs EV1A and EV2A. CKO rhythms under these conditions showed significantly reduced period and amplitude compared with wild‐type (WT) controls, but persisted for > 2 weeks, consistent with these mice possessing a residual endogenous biological oscillation that is not entrained by standard environmental light:dark cycles (Fig EV2, EV3, EV4, EV5 and Appendix). In support of this interpretation, and in accordance with previous reports (Maywood et al, 2011; Ono et al, 2013b), longitudinal bioluminescence recordings of organotypic PER2::LUC SCN slices cultured ex vivo from WT or CKO neonates revealed rhythmic PER2 expression in approximately 40% of CKO slices (Fig 1C). In line with behavioural data and previous reports, these CKO SCN rhythms exhibited significantly shorter periods compared with WT controls (Fig 1D).
Figure 1. CRY‐independent circadian timekeeping occurs cell‐autonomously.
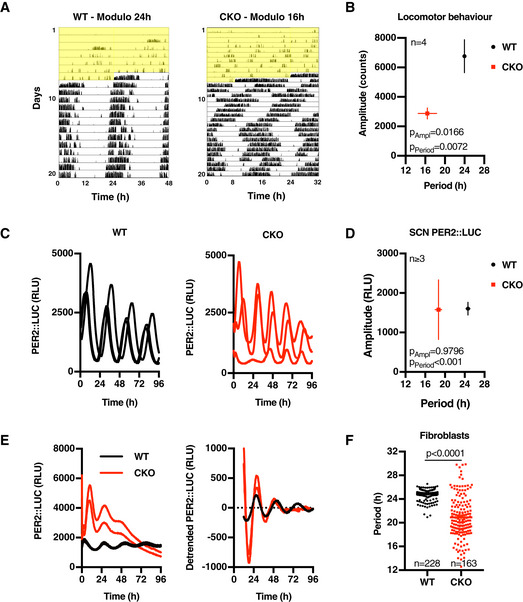
- Representative double‐plotted actograms showing wheel‐running activity of wild‐type (WT) and CRY‐deficient (CRY knockout; CKO) mice during constant light (yellow shading) and thereafter in constant darkness. Note the 48 h x‐axis for WT vs. 32 h for CKO. Full figure showing CKO data in modulo 24 h is presented in Fig EV1A.
- Mean period and amplitude (± SEM) of mouse behavioural data (n = 4). P‐values were calculated by two‐way ANOVA.
- Longitudinal bioluminescence recordings of organotypic SCN slices from WT (black) and CKO (red) PER2::LUC mice (RLU; relative light units).
- Mean period and amplitude (± SEM) of rhythmic SCN bioluminescence traces. P‐values were calculated by two‐way ANOVA.
- Circadian PER2::LUC expression in immortalised WT and CKO adult lung fibroblasts. Left panel shows two raw traces of a representative longitudinal bioluminescence recording, and right panel shows same data detrended with a 24‐h moving average to remove differences in baseline expression.
- Period of rhythmic fibroblast bioluminescence traces from at least 31 experiments (n ≥ 3 per experiment, individual values ± SEM shown). P‐values were calculated by an unpaired t‐test with Welch correction. Standard deviations differ significantly between WT and CKO (F‐test: P < 0.0001).
Figure EV1. CRY‐independent circadian timekeeping occurs cell‐autonomously.
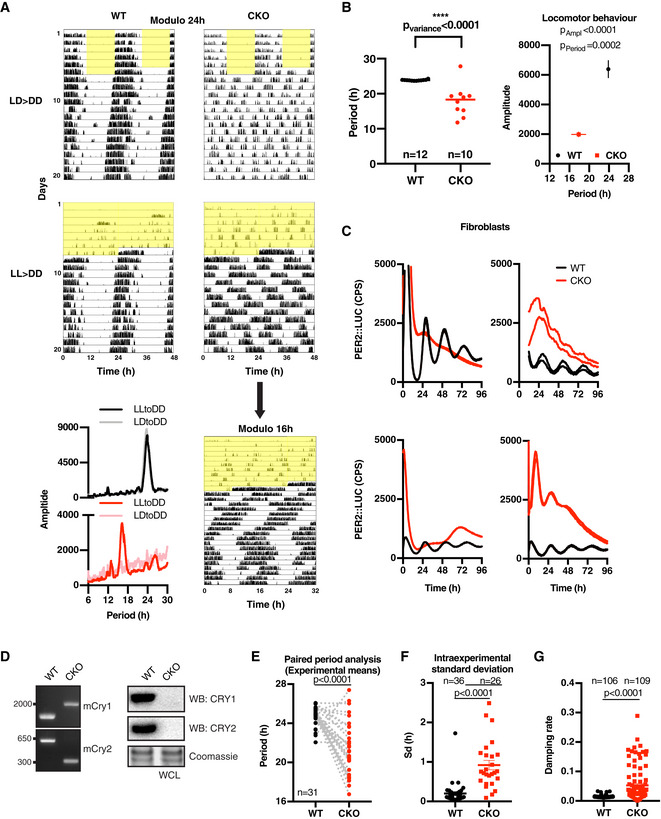
- Representative double‐plotted actograms showing wheel‐running activity of WT and CKO mice during 12 h:12 h light:dark (LD) cycles (yellow shading indicating lights on) (top) or during constant light (LL) (bottom) and thereafter in constant darkness (DD). Top four figures have same x‐axis (modulo 24 h). Rhythmic behaviour of CKO mice in LL > DD condition becomes clear when plotting the data in 16‐h modulo (i.e. x‐axis being 32 h). LD figure WT modulo 24‐h and CKO modulo 16‐h are also presented in Fig 1A. Bottom left, representative periodograms of WT and CKO mice over 2 weeks in constant darkness, following either 12 h:12 h light:dark cycles or constant light.
- A second experimental cohort highlights significant differences in period variance (left, horizontal line represents mean, F‐test for variance); period and amplitude (right) of CKO (n = 10) vs. WT (n = 12) mice over 2 weeks in constant darkness following 1 week under constant light (mean ± SEM, 2‐way ANOVA with Sidak’s MCT).
- Examples of independent bioluminescence recordings of PER2::LUC expression in CRY‐deficient fibroblasts showing variability in shape and baseline of rhythmic CKO traces. Two representative traces are shown per experiment. Stringent entrainment, e.g. with temperature cycles or dexamethasone, increases the likelihood of observing rhythmicity, but only in approximately 30% of the experiments did we observe clearly rhythmic expression of PER2::LUC over 3 cycles. Despite our best efforts, over many years, we were unable to identify a set of entrainment and recording conditions that consistently produced CKO PER2::LUC rhythms and we were forced to conclude that more variables were at play than we were adequately able to control for.
- Genotyping CKO fibroblasts used throughout this study. Left: PCR genotyping shows the expected pattern of CRY1 and CRY2 knockout. Right: Western blot analysis of whole cell lysates (WCL) and probed with antibodies against CRY1 and CRY2.
- Interexperimental comparison of PER2::LUC periods in WT vs. CKO fibroblasts. Paired comparison of period means of experiments used for Fig 1F where CKO traces were rhythmic. P‐values were calculated by paired t‐test.
- Intraexperimental standard deviations (i.e. between replicates) were calculated for all experiments with > 3 rhythmic traces (mean ± SEM). P‐value was calculated by unpaired t‐test.
- Damping rates of all individual detrended traces of example experiments shown in Fig EV1E were calculated by damped sine wave fitting (mean ± SEM, WT n = 106, CKO n = 109). P‐value was calculated by unpaired t‐test with Welch correction.
Source data are available online for this figure.
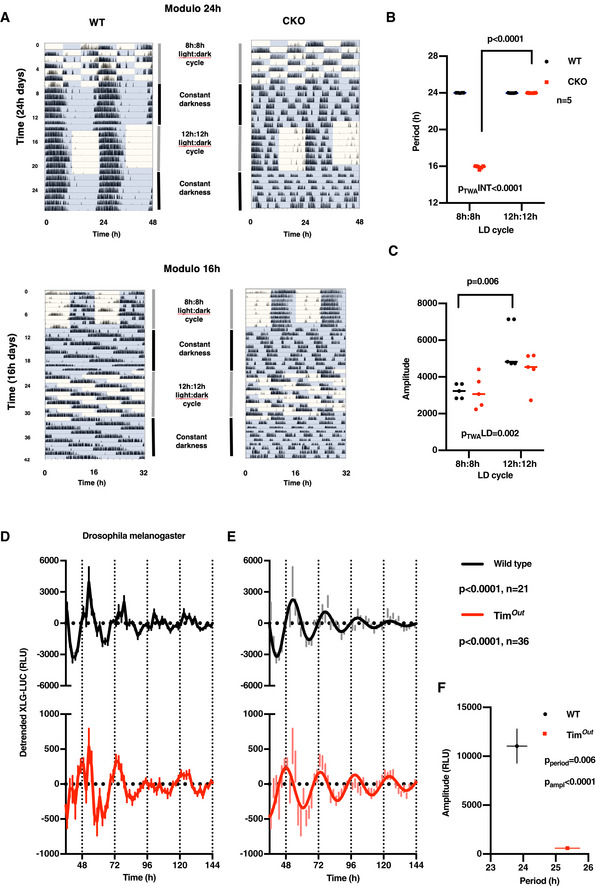
Figure EV2. Entrainment deficiency of CRY‐deficient mice to environmental light:dark cycles and Timeless‐independent protein rhythms in Drosophila melanogaster .
-
ARepresentative actograms showing that CKO mice (n = 5) cannot be entrained to 8 h:8 h or 12 h:12 h light:dark cycles, whereas WT mice entrain to 12 h:12 h but not 8 h:8 h light‐dark cycles (n = 5). Equal numbers of age‐matched male and female mice were used.
-
B, CFor CKO mice during under LD cycles, the dominant period of behavioural rhythms is determined by the period of the zeitgeber, whereas amplitude does not vary significantly between 16‐h and 24‐h cycles. In contrast, the period of WT behavioural rhythms does not vary significantly between 16‐h and 24‐h cycles, whereas amplitude is significantly reduced under unnatural 8 h:8 h light:dark cycles. This indicates the robustness conferred by CRY to circadian rhythms of locomotor activity in WT mice in vivo. Two‐way ANOVA P‐values and significance from Sidak’s multiple comparisons test are reported; horizontal line represents mean.
-
DNormalised, detrended bioluminescence recording of the XLG‐luciferase reporter (XLG‐LUC; equivalent of PER2::LUC) expressed in WT and timeless knockout (Timout) flies under constant darkness (detrended means ± SEM; WT n = 21, Timout n = 36). Note the difference in y‐axis scaling.
-
EDamped sine wave fit to the data presented in (D). P‐values (extra sum‐of‐squares F‐test) indicate comparison of fit test with the null hypothesis (straight line).
-
FSignificant differences in the period and amplitude of XLG‐LUC rhythms of Timout compared with WT flies. Mean ± SEM, P‐values indicate unpaired t‐test, WT n = 21, Timout n = 36.
Data information: The generation of Timout flies is reported in Lamaze et al (2017). Similar to CRY‐deficient mice, whole gene timeless knockout flies are characterised as being behaviourally arrhythmic under constant darkness following entrainment by light:dark cycles: https://opus.bibliothek.uni‐wuerzburg.de/frontdoor/index/index/year/2015/docId/11914
Figure EV3. CRY‐independent rhythms are regulated post‐transcriptionally.
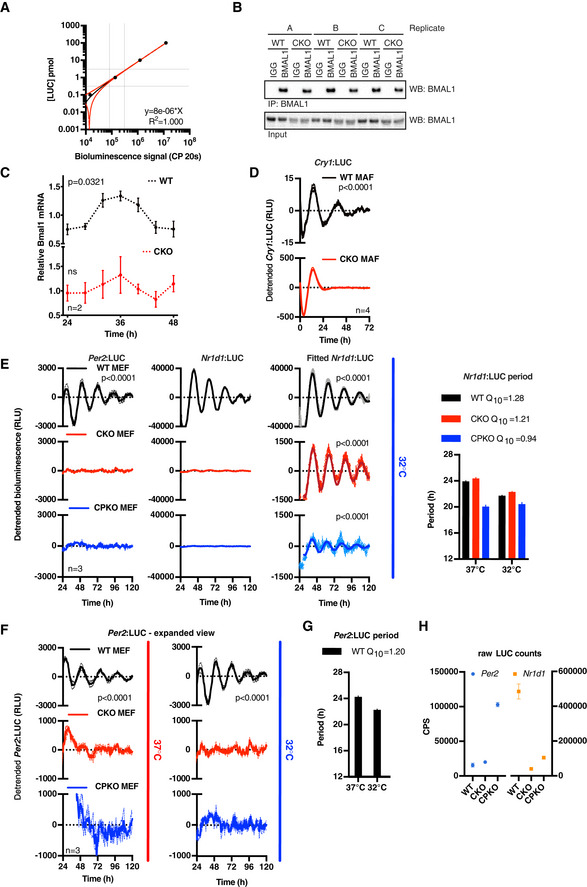
- Standard curve of recombinant luciferase that was used to determine the number of PER2::LUC molecules. Known concentrations of recombinant luciferase were spiked into (non‐luciferase containing) cell lysate to reproduce experimental conditions, and the luciferase signal was measured with a 20‐s integration time (CP 20 s; counts per 20 s). Data were fitted with a straight line (red line, ± 95% CI). The grey dotted lines indicate the (linear) area of the curve used to determine the number of PER2::LUC molecules of the experiment shown in Fig 3A.
- Bmal1 mRNA levels were determined by qPCR over one circadian period (n = 2, mean ± SEM). The WT timeseries were preferentially fit with a circadian damped sine wave compared with a straight line (extra sum‐of‐squares F‐test, P = 0.0321), but not the CKO timeseries (ns). PER2::LUC co‐recording from parallel cultures is depicted in Fig 3C.
- Detrended bioluminescence data of transcriptional reporter Cry1:LUC in WT and CKO mouse adult fibroblasts (MAFs) (n = 4, mean ± SEM). WT traces fit circadian damped sine wave over straight line in extra sum‐of‐squares F‐test (P < 0.0001), whereas no sine wave could be fit to CKO traces (no P‐value).
- Detrended Per2 and Nr1d1 promoter activity in WT, CKO and quadruple Cry1/2‐Per1/2 knockout (CPKO) mouse embryonic fibroblasts (MEFs) recorded at 32°C, n = 3, mean (solid) ±SEM (dashed). WT Per2 and all Nr1d1 traces were preferentially fit with a circadian damped sine wave over straight line (extra sum‐of‐squares F‐test, P < 0.0001), whereas no sine wave could be fit to the other traces (not significant, no P‐value). Period analysis shows that Nr1d1 promoter oscillations are temperature‐compensated (mean ± SEM, n = 3).
- Expanded view of Per2:LUC recordings to show no circadian oscillations of Per2 promoter activity is detected in C(P)KO MEFs, n = 3, mean (solid) ±SEM (dashed). Zoomed‐out version of 37°C recording is also shown in Fig 3D and of 32°C recording in Fig EV3E. WT Per2 traces were preferentially fit with a circadian damped sine wave over straight line (extra sum‐of‐squares F‐test, P < 0.0001), whereas no sine wave could be fit to the other traces (not significant, no P‐value).
- Period analysis shows that also WT Per2 promoter oscillations are temperature‐compensated, as expected (n = 3, mean ± SEM).
- 24‐h average (from 24 to 48 h) raw luciferase counts from Per2:LUC and Nr1d1:LUC counts demonstrate that reporter expression levels do not explain a difference in amplitude in circadian oscillations (n = 3, mean ± SEM).
Source data are available online for this figure.
Figure EV4. PER2::LUC stability oscillates in CRY‐deficient cells.
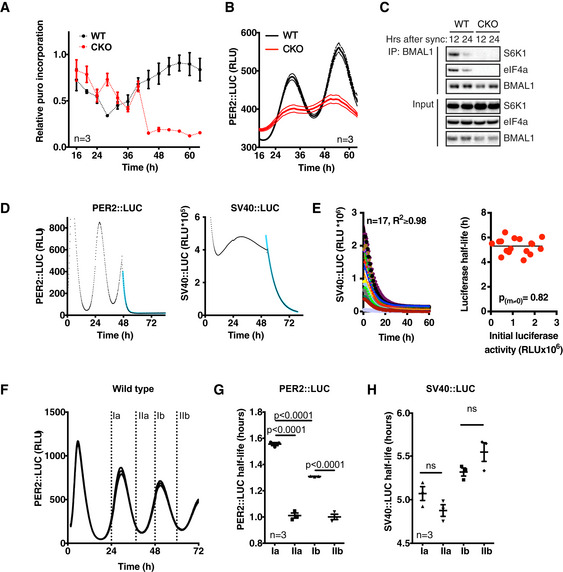
- WT and CKO cells were assayed for puromycin incorporation over two circadian cycles. Cells were synchronised by temperature cycles and dexamethasone, and harvested every 3 h after a 10‐min puromycin pulse (10 µg/ml). Incorporation was measured by Western blotting with an anti‐puromycin antibody. Western blots were quantified and corrected for total protein loading (Coomassie staining). Mean ± SEM (n = 3).
- Bioluminescence co‐recording of puromycin labelling time course shows circadian PER2::LUC expression in both genotypes. Mean ± SEM (n = 3).
- Western blot analysis of BMAL1 immunoprecipitation with antibodies specific for S6K, eIF4a and BMAL1. Cells were harvested 12 or 24 h after dexamethasone synchronisation, and BMAL1 was immunoprecipitated.
- Example of a bioluminescence recording of WT PER2::LUC (left) or SV40::LUC (right) cells pulsed with 10 µM CHX after 46 h of recording. The resulting raw data (symbols) were fitted with a one‐phase decay curve (blue line).
- Multiple stable SV40::LUC fibroblast lines with different basal expression levels were treated with 25 μg/ml CHX allowing the turnover of luciferase to be inferred from the decay in bioluminescence signal. Left panel, the decline in luciferase activity was fit with a simple one‐phase exponential decay curve (solid lines) to derive the half‐life of luciferase in each cell line. Right panel, no significant relationship between the level of luciferase expression and luciferase stability was observed (straight line vs. horizontal line fit P‐value is reported, extra sum‐of‐squares F‐test).
- Timing of CHX pulses (labelled I‐II a (cycle 1) and b (cycle 2)), plotted on bioluminescence trace of WT PER2::LUC control cells, corresponding to data presented in G and H.
- PER2::LUC half‐life at different phases in the circadian cycle in WT cells (mean ± SEM). P‐values were calculated by two‐tailed t‐test. Half‐life was quantified by one‐phase decay line‐fitting of bioluminescence traces from CHX pulsed cells.
- SV40::LUC half‐life at different phases in the circadian cycle in fibroblasts (mean ± SEM). P‐values were calculated by two‐tailed t‐test.
Source data are available online for this figure.
Figure EV5. A role for CK1 and GSK3 in the cytoplasmic oscillator.
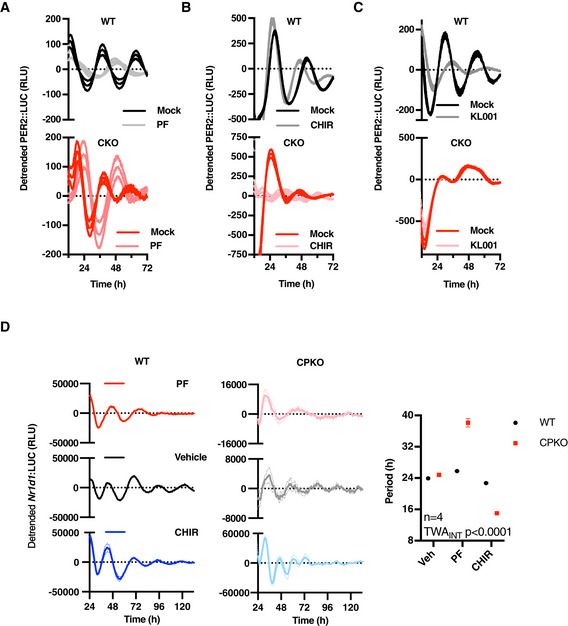
- Bioluminescence recordings of WT and CKO PER2::LUC cells in the presence or absence of CK1δ/ε inhibitor PF670462 (0.3 µM; PF), as quantified in Fig 5A (n = 3, detrended mean ± SEM).
- As in (A), GSK3 inhibitor CHIR99021 (5 µM; CHIR).
- As in (A), in the presence of CRY turnover inhibitor KL001 (1 µM).
- Bioluminescence recordings and respective period quantifications of WT and CPKO Nr1d1::LUC cells in the presence or absence of CK1δ/ε inhibitor PF670462 (0.1 µM) or GSK3 inhibitor CHIR99021 (3 µM; CHIR) (n = 4, detrended mean ± SEM). P‐value of the two‐way ANOVA (genotype vs. drug interaction effect) is reported.
Two explanations might account for the variable CKO SCN phenotype: (i) the previously proposed explanation: genetic loss of function is compensated at a network level by SCN‐specific neuronal circuits whose function is sensitive to developmental phase and small variations in slice preparation (Liu et al, 2007; Evans et al, 2012; Ono et al, 2013b; Tokuda et al, 2015); or (ii) CKO (SCN) cells have cell‐intrinsic circadian rhythms that are expressed (or observed) more stochastically and with less robustness than their WT counterparts, and can be amplified by SCN interneuronal signalling (Welsh et al, 2010; O’Neill & Reddy, 2012).
To distinguish between these two possibilities, we asked whether PER2::LUC rhythms are observed in populations of immortalised PER2::LUC CKO adult fibroblasts, which lack the specialised interneuronal neuropeptidergic signalling that is so essential to SCN amplitude and robustness in and ex vivo (Welsh et al, 2010; O’Neill & Reddy, 2012). We observed this to be the case (Figs 1E, and EV1C and D). Across > 100 recordings, using independently generated cell lines cultured from multiple CRY‐deficient mice (male and female), we observed PER2::LUC rhythms that persisted for several days under constant conditions. Again, the mean period of rhythms in CRY‐deficient cells was significantly shorter than WT controls, and with increased variance within and between experiments (F‐test P‐value < 0.0001, Figs 1F, and EV1E and F). Consistent with SCN results, rhythmic PER2::LUC expression in CKO cells occurred stochastically between experiments, being observed in ~ 30% of independently performed assays. Importantly, there was very little variation in the occurrence of rhythmicity within experiments meaning that in any given recording all CKO replicate cultures were rhythmic or none, whereas WT cultures were always rhythmic. CKO PER2::LUC rhythms damped more rapidly than wild‐type controls (Fig EV1G), and were more sensitive to acute changes in temperature than WT controls (Fig 2A and C), consistent with their oscillation being less robust. Crucially though, the PER2::LUC rhythms in CKO cells were temperature‐compensated (Fig 2A and B) and entrained to 12 h:12 h 32°C:37°C temperature cycles in the same phase as WT controls (Fig 2C), and thus conform to the classic definition of a circadian rhythm (Pittendrigh, 1960), which does not stipulate any lower threshold for amplitude or robustness.
Figure 2. CRY‐less oscillations are temperature‐compensated and entrained.
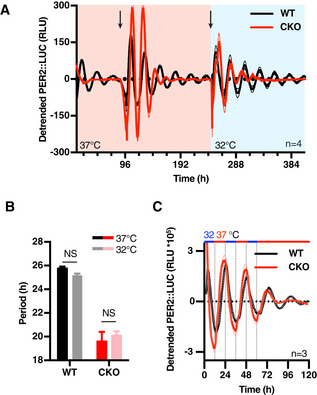
- Detrended traces of bioluminescence recordings of WT and CKO fibroblast at different constant temperature conditions within the physiological range (n = 4, solid lines: mean, dashed lines: ± SEM). Temperature was changed from 37°C to 32°C halfway through the experiment, as depicted by red/blue shading. Arrows represent medium changes. Note the lack of rhythmicity in the first 3 days in CKO and the appearance of rhythmicity after the first medium change.
- Quantification of period from recordings presented in (A). Both WT and CKO oscillations are temperature‐compensated with respective Q10s of 1.05 and 0.95 (n = 3, mean ± SEM). P‐values were determined by two‐tailed t‐test.
- Bioluminescence of WT and CKO PER2::LUC cells during temperature entrainment (12 h 32°C (blue)–12 h 37°C (red)) (n = 3, solid lines: mean, dashed lines: ± SEM).
Clearly then, the bona fide circadian rhythms we observed in cultured CKO cells are insufficiently robust to facilitate entrainment of ~ 24‐h rest–activity cycles that are a classic hallmark of circadian rhythms in vivo. This suggests that CRY‐dependent transcriptional feedback repression confers robustness to rhythmic cellular clock output that is required for circadian organisation of overt behaviour, rather than generating circadian rhythms per se. To test this in another model system, we turned to Drosophila melanogaster, where Timeless fulfils the functionally analogous role to mammalian CRY proteins as the obligate partner of Period, required for repression of circadian transcription at E‐box promotor elements, and is required for the circadian organisation of locomotor activity under constant darkness (Sehgal et al, 1994, 1995). In assays of Period::LUC (XLG‐LUC) activity in freely behaving flies, we observed circadian bioluminescence rhythms in timeless knockout animals that were significantly longer in period than WT controls (Fig EV2, EV3, EV4, EV5). As observed for CRY‐deficient cells, rhythms in Timeless‐deficient flies persisted over several days with much lower amplitude than WT flies.
In contemporary models of the mammalian cellular clockwork, CRY proteins are essential for rhythmic PER protein production; however, the stability and activity of PER proteins are also regulated post‐translationally (Iitaka et al, 2005; Lee et al, 2009; Philpott et al, 2020). Considering recent reports that there is no obligate relationship between clock protein turnover and circadian regulation of its activity in the fungus Neurospora crassa (Larrondo et al, 2015), that nascent transcription is not required for circadian rhythms in the green lineage (O’Neill et al, 2011), or in isolated human red blood cells (O’Neill & Reddy, 2011), we next investigated the relative contribution of transcriptional vs. post‐translational regulation to circadian PER2::LUC rhythms in CRY‐deficient cells.
CRY‐independent PER2::LUC rhythms are driven by a non‐transcriptional process
CRY has previously been described as the driving factor for feedback repression of BMAL1/CLOCK‐dependent transcriptional activation, and is therefore considered essential to the rhythmic regulation of clock‐controlled genes (CCGs). In fact, overexpression studies have suggested PER requires CRY to exert its function as a BMAL1‐CLOCK repressor (Ye et al, 2014; Chiou et al, 2016). This importance of CRY for BMAL1‐CLOCK repression (and auto‐repression of Cry and Per) was also suggested by the increased PER2::LUC levels observed in CKO cells (Figs 1E and EV1C). Indeed, at the peak of PER2::LUC expression, CKO cells contain approximately twice as many PER2 molecules compared with their WT counterparts (Figs 3A and EV3A).
Figure 3. CRY‐independent rhythms are regulated post‐transcriptionally.
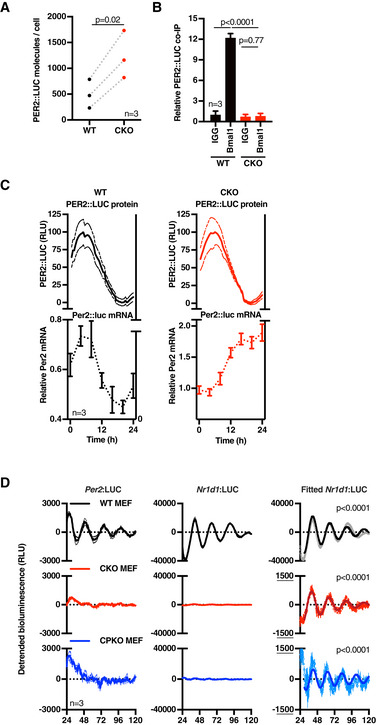
- Mean number of PER2::LUC molecules per cell at the estimated peak of PER2 expression for each cell line (mean of three experiments, n = 3 each). P‐values were calculated by paired t‐test.
- PER2::LUC binding to BMAL1 in WT and CKO cells. Cells were harvested at the peak of PER2 expression, BMAL1 was immunoprecipitated, and PER2::LUC binding was measured by bioluminescence measurements (n = 3, mean ± SD). P‐values were calculated by unpaired t‐test.
- Per2 mRNA levels in WT (left) and CKO (right) cells were determined by qPCR over one circadian cycle (bottom), whilst PER2::LUC bioluminescence (top, min‐max normalised) was recorded from parallel cultures. Per2 mRNA reported relative to Rns18s (bottom), n = 3, ± SEM; PER2::LUC (top) presented as mean (solid) ± SEM (dashed), n = 3. The WT mRNA trace was preferentially fit by a circadian damped sine wave compared with straight line (P = 0.0412, extra sum‐of‐square F‐test), whereas CKO data were not (ns).
- Detrended Per2 and Nr1d1 promoter activity in WT, CKO and quadruple Cry1/2‐Per1/2 knockout (CPKO) mouse embryonic fibroblasts (MEFs) recorded at 37°C, n = 3, mean (solid) ±SEM (dashed). Nr1d1 data were preferentially fit with a circadian damped sine wave over straight line (P < 0.0001, extra sum‐of‐squares F‐test) (right hand graphs, solid lines; error bars, SEM). Similar recordings performed at 32°C and an expanded view of Per2 data are presented in Fig EV3E and F.
Although not sufficient to completely rescue rhythms in CKO cells, it seemed plausible that increased PER or other clock protein expression might partially compensate for the loss of CRY function and continue to exert auto‐regulation through rhythmic BMAL1‐CLOCK binding, thereby accounting for the residual PER2::LUC rhythms in CKO cells. To test this possibility, we compared BMAL1‐PER2 binding at the expected peak of BMAL1‐PER2 complex formation (i.e. at the peak of PER2::LUC expression) in WT and CKO cells. To this end, we immunoprecipitated BMAL1 and measured the associated PER2::LUC activity. In accordance with CRY being required for PER2‐BMAL1 binding, we did not find a PER2::LUC‐BMAL1 complex in CKO cells, whilst the complex was readily detected in WT cells (Figs 3B, and EV3B and I), strongly suggesting that residual oscillations in PER2::LUC cannot result from a residual negative feedback upon the BMAL1‐CLOCK complex.
In the absence of PER:CRY‐mediated feedback repression, it seemed unlikely that CRY‐independent oscillations in PER2::LUC expression are driven directly by rhythms in Per2 transcription. Indeed, whereas PER2::LUC in co‐recorded cells showed a clear variation over 24 h, Per2 mRNA in parallel replicate CKO cultures instead exhibited a gradual accumulation (Fig 3C). In contrast and as expected (Feeney et al, 2016a), Per2 mRNA in WT cells varied in phase with co‐recorded PER2::LUC oscillations. The gradual increase of Per2 mRNA in CKO cells is concordant with Per2 transcriptional derepression predicted by the canonical TTFL model, accounting for the generally increased levels of PER2::LUC we observed (Fig 3A), but not their oscillation. In agreement with these findings and in contrast with WT cells, Bmal1 mRNA also showed no significant variation in CKO cells (Fig EV3C), suggesting that E‐box‐dependent circadian regulation of REV‐ERB activity may not occur in the absence of CRY‐mediated feedback repression. In an independent validation, we assessed the activity of the circadian E‐box‐driven Cry1‐promoter (Maywood et al, 2013) in mouse adult WT and CKO lung fibroblasts (MAFs) (Fig EV3D), as well as the Per2‐ and Rev‐erb α‐ (Nr1d1‐) promoters in mouse embryonic fibroblasts (MEFs) (Figs 3D and EV3E–H). No rhythmic Cry1‐ or Per2‐promoter activity was observed in either set of CKO cells under any condition, whereas isogenic control cells showed clear circadian regulation of these promoters.
In recordings from Nr1d1:LUC MEFs however, we were most surprised to observe temperature‐compensated circadian rhythms in the activity of the Nr1d1 promoter in CKO cells, at just ~ 3% amplitude of WT cells, that persisted for several days (Figs 3D and EV3E, red traces). In the same experiments, similar but still noisier and lower amplitude rhythms were also detected in quadruple knockout MEFs that were also deficient for PER1/2, as well as CRY1/2 (CPKO, Figs 3D and EV3E, blue traces), confirming these oscillations cannot be attributable to any vestigial activity of PER proteins. We acknowledge it is conceivable that some unknown TTFL‐type mechanism might generate these residual oscillations in Nr1d1 promoter activity. However, we find it more plausible that residual oscillations of Nr1d1:LUC in CKO cells are the output of a post‐translational timekeeping mechanism, from which the amplification and robustness conferred by CRY‐dependent transcriptional feedback repression has been subtracted. Indeed, we note that besides CRY, Nr1d1 expression is regulated by many other transcription factors, e.g. AP‐1, NRF2, NF‐KB and BMAL1/CLOCK (Preitner et al, 2002; Yang et al, 2014; Wible et al, 2018), whose activity is regulated post‐translationally by the same rather promiscuous kinases that rhythmically regulate PER and BMAL1 in other contexts (Eide et al, 2002; Iitaka et al, 2005; Sahar et al, 2010; Narasimamurthy et al, 2018), e.g. casein kinase 1 and glycogen synthase kinase (Preitner et al, 2002; Liang & Chuang, 2006; Tullai et al, 2011; Rada et al, 2011; Medunjanin et al, 2016; Jiang et al, 2018).
Circadian control of PER2 stability persists in the absence of CRY
The concentrations of luciferase substrates (Mg.ATP, luciferin, O2) under our assay conditions are > 10× higher than their respective K m (Feeney et al, 2016a) and so it is implausible that PER2::LUC rhythms in CKO cells result from anything other than circadian regulation in the abundance of the PER2::LUC fusion protein. Indeed, PER2::LUC levels measured in cell lysates perfectly mirrored longitudinal PER2::LUC recordings from both WT and CKO cells (Fig 4A). We observed that the addition of the proteasomal inhibitor MG132 to asynchronous cells led to acute increases in PER2::LUC levels which were significantly greater in CKO cells than in WT controls, indicating that CKO cells support higher basal rates of PER2 turnover (Fig 4B and C). In consequence therefore, relatively small changes in the rate of PER2::LUC translation or degradation should be sufficient to affect the steady state PER2::LUC concentration. CKO cells exhibit no rhythm in Per2 mRNA (Fig 3C and D), nor do they show a rhythm in global translational rate (Fig EV4A and B), nor did we observe any interaction between BMAL1 and S6K/eIF4 as occurs in WT cells (Lipton et al, 2015; Fig EV4C). We therefore investigated whether changes in PER2::LUC stability might be responsible for the persistent bioluminescence rhythms in CKO cells, by analysing the decay kinetics of luciferase activity during saturating translational inhibition.
Figure 4. PER2::LUC stability oscillates in CRY‐deficient cells.
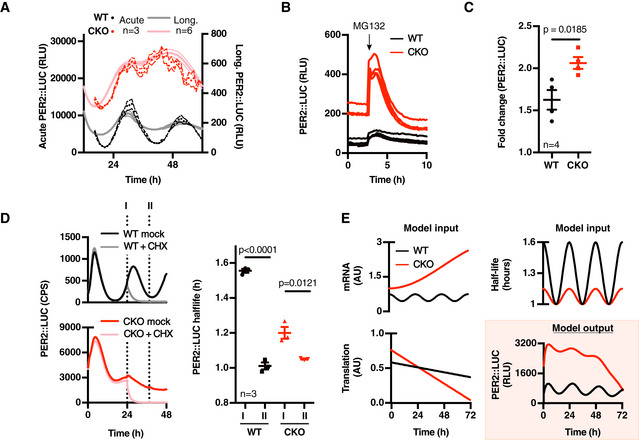
- Actual PER2::LUC levels (dark symbols (3‐h moving average, n = 3 ± SEM, 4 outliers removed)) as assayed in acute luciferase assays on cell lysates from cells harvested every hour over 48 h, compared with parallel longitudinal co‐recordings from cells in the presence of 0.1 mM luciferin (light lines (n = 6, mean ± SEM)).
- PER2::LUC recording of asynchronous WT and CKO cells pulsed with proteasome inhibitor MG132 (10 µM, applied at the arrow) (n = 3, mean ± SEM).
- Quantification of relative PER2::LUC induction upon proteasome inhibition, n = 3, mean (solid) ± SEM (dashed). P‐value was calculated by unpaired t‐test.
- Phase‐dependent PER2::LUC half‐life was determined by inhibiting translation at different circadian phases and fitting the resulting data with a one‐phase exponential decay curve (n = 3, mean ± SEM). Left image depicts the timing of cycloheximide (CHX, 10 µM) pulses (labelled I (PER2 levels going up) and II (PER2 levels going down)), plotted on PER2::LUC bioluminescence traces of control cells (dark colours). A representative trace of CHX‐treated cells at time point I is shown in light colours. See Fig EV4D and F for more raw data and time points. Right image shows quantifications, and P‐values were calculated by unpaired t‐test.
- A simple model incorporating mRNA, protein translation and PER2::LUC stability that were measured experimentally (inputs) shows that the observed oscillating stability of PER2 is sufficient to generate rhythmic PER2::LUC expression (output).
In the presence of 10 μM cycloheximide (CHX), PER2::LUC bioluminescence decayed exponentially (Figs 4D and EV4D, R 2 > 0.9), with a half‐life that was consistently < 2 h (Figs 4D and EV4D, F and G); much less than the half‐life of luciferase expressed in fibroblasts under a constitutive promoter (≥ 5 h, Fig EV4D, E and H). Moreover, we observed a significant variation (±50%) in the half‐life of PER2::LUC between the rising and falling phases of its expression (1.5 vs. 1 h, respectively, Figs 4D and EV4G) without any commensurate change in global protein turnover (Fig EV4H). Strikingly, we also observed a similar phase‐dependent variation of PER2::LUC stability in CKO cells, with a smaller (± 20%) but significant difference between opposite phases of the oscillation (Fig 4D). To test if a 20% variation in protein half‐life, in the absence of any underlying mRNA abundance rhythm, was sufficient to account for our experimental observations given the intrinsically high turnover of PER2, we made a simple mathematical model using experimentally derived values for mRNA level, protein half‐life and translation (Figs 3C and EV4). We found that the model produced PER2::LUC levels that closely approximate our experimental observations (Fig 4E). Thus whilst we cannot absolutely discount the possibility that rhythmic translation contributes to the PER2::LUC rhythms in CKO cells, we found no evidence to support this, whereas experimental observations and theoretical modelling do suggest rhythmic PER2 degradation alone is sufficient to explain the residual bioluminescence rhythms we observe in CKO PER2::LUC fibroblasts.
CK1δ/ε and GSK3 contribute to CRY‐independent PER2 oscillations
PER2 stability is primarily regulated through phosphorylation by casein kinases (CK) 1δ and 1ε, which phosphorylate PER2 at phosphodegron sites to target it for proteasomal degradation (Lee et al, 2009; Philpott et al, 2020). In this context, CK1δ/ε frequently operates in tandem with glycogen synthase kinase (GSK) 3α/β, as occurs in the regulation of β‐catenin stability (O’Neill et al, 2013; Robertson et al, 2018). Interestingly, both CK1δ/ε and GSK3α/β have a conserved role in determining the speed at which the eukaryotic cellular circadian clock runs (Hastings et al, 2008; Causton et al, 2015), both in the presence and absence of transcription (Hirota et al, 2008; Meng et al, 2008; O’Neill et al, 2011; Beale et al, 2019). This is despite the fact that the clock proteins phosphorylated by these kinases are highly dissimilar between animals, plants and fungi (Causton et al, 2015; Wong & O’Neill, 2018).
We hypothesised that the PER2::LUC rhythm in CKO cells reflects the continued activity of a post‐translational timekeeping mechanism that involves CK1δ/ε and GSK3α/β, which results in the differential phosphorylation and turnover of clock protein substrate effectors such as PER2 during each circadian cycle (O’Neill et al, 2013). To test this, we incubated WT and CKO cells with selective pharmacological inhibitors of CK1δ/ε (PF670462; PF) and GSK3α/β (CHIR99021; CHIR), which have previously been shown to slow down, and accelerate, respectively, the speed at which the cellular clock runs in a wide range of model organisms (Badura et al, 2007; Hirota et al, 2008; O’Neill et al, 2011; Causton et al, 2015). As a control we used KL001, a small molecule inhibitor of CRY degradation (Hirota et al, 2012), which has previously been shown to affect cellular rhythms in WT cells via increased CRY stability.
We found that inhibition of CK1δ/ε and GSK3‐α/β had the same effect on circadian period in CKO cells, CPKO cells and WT controls (Figs 5A and B, and EV5A, B and D). In contrast, KL001 increased period length and reduced amplitude of PER2::LUC expression in WT cells but had no significant effect on post‐translationally regulated PER2::LUC rhythms in CKO cells (Figs 5C and EV5C). Besides confirming the specific mode of action for KL001 in targeting CRY stability, these observations implicate CK1δ/ε and GSK3α/β in regulating the post‐translational rhythm reported by PER2::LUC in CKO cells.
Figure 5. A role for CK1 and GSK3 in the cytoplasmic oscillator.
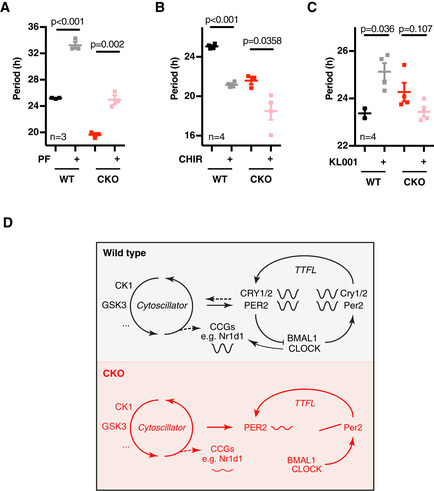
- Period (n = 3, mean ± SEM) analyses of WT and CKO PER2::LUC cells in the presence or absence of CK1δ/ε inhibitor PF670462 (0.3 µM; PF). P‐values were calculated by unpaired t‐test.
- As in (A, n = 4), GSK3 inhibitor CHIR99021 (5 µM; CHIR).
- As in (A, n = 4), in the presence of CRY inhibitor KL001 (1 µM).
- Schematic model integrating CRY‐independent timekeeping into the existing canonical model of the circadian clock. The CRY‐dependent gene expression feedback loop (TTFL) is required for most circadian regulation of transcriptional clock‐controlled genes (CCGs) and therefore for robustness and behavioural and physiological rhythmicity. However, it is dispensable for circadian timekeeping per se, as reported by residual oscillations in PER2 protein levels, suggestive of the existence of a coupled underlying (cytosolic) timekeeping mechanism involving CK1 and GSK3 (cytoscillator).
Data information: See Fig EV5 for raw data.
Discussion
We found that CRY‐mediated transcriptional feedback in the canonical TTFL clock model is dispensable for cell‐autonomous circadian timekeeping in cellular models. Whilst we cannot exclude the possibility that in the SCN, but not fibroblasts, PER alone may be competent to effect transcriptional feedback repression in the absence of CRY, we are not aware of any evidence that would render this possibility biochemically feasible.
Circadian rhythms of PER abundance were observed in CKO SCN slices and fibroblasts, as well as Timeless‐deficient flies, indicating that the post‐translational mechanisms that normally confer circadian rhythmicity onto PER proteins in WT cells remain ostensibly intact in the absence of canonical transcriptional feedback repression. Importantly however, CKO PER2 rhythms were only observed in a minority of recordings (~ 30%), and when observed, they showed increased variance of period and sensitivity to perturbation. This reduced capacity to perform without failure under a wide range of conditions means that CRY‐deficient oscillations are less robust than those in WT cells (Merriam‐Webster Dictionary, 2020), and the reduced robustness of oscillations may explain or contribute to the obvious impairment of circadian physiological organisation at the organismal scale. We were unable to identify all of the variables that contribute to the apparent stochasticity of CKO PER2::LUC oscillations, and so cannot distinguish whether this variability arises from reduced fidelity of PER2::LUC as a circadian reporter or impaired timing function in CKO cells. In consequence, we restricted our study to those recordings in which clear bioluminescence rhythms were observed, enabling the interrogation of TTFL‐independent cellular timekeeping.
In the field of chronobiology, CKO cells and mice are often used as clock‐deficient models. Indeed, canonical circadian transcriptional output is essentially absent from these models (Hoyle et al, 2017; Ode et al, 2017), and thus for studying TTFL‐mediated control of overt physiology, they are appropriate negative controls. However, as the underlying timekeeping mechanism seems at least partially intact, we consider it inappropriate to describe CKO cellular models as arrhythmic. Indeed, rest/activity behaviour of CKO mice does entrain to daily cycles of restricted feeding (Iijima et al, 2005), which is SCN‐independent (Storch & Weitz, 2009). We also observed (about) daily rest–activity cycles in vivo and SCN PER2::LUC rhythms ex vivo that, whilst being 20–30% shorter and less robust than WT controls, suggests CRY‐independent timing mechanisms can co‐ordinate communication between cells under some conditions. Thus, non‐TTFL‐mediated timekeeping seems sufficient to serve as an almost daily interval timer in cells and in vivo (Crosby et al, 2019), but rhythmic precision, robustness and physiological function require the participation of CRY‐dependent processes.
Previous studies have reported isolated CKO cells to be entirely arrhythmic (Sato et al, 2006; Ukai‐Tadenuma et al, 2011; Ode et al, 2017), in stark contradiction with our findings (see also technical discussion). However, most such studies measured changes in transcription either by quantitative RT–PCR, or with luciferase fusions to fragments of the Bmal1, Per and Cry promoters which we also found to be arrhythmic in CKO cells. We did observe low amplitude oscillations in Nr1d1 promoter activity, however. It may be pertinent to report that our MEF recordings only revealed circadian oscillations in Nr1d1‐promoter activity, and only in bicarbonate‐buffered medium supplemented with 1 mM luciferin and 10% serum (Fig 3D), but not in low serum or HEPES‐buffered media, as employed in other studies that used different circadian reporters and may have employed sub‐saturating concentrations of luciferin (Feeney et al, 2016a). It is also plausible that the high sensitivity of the electron‐multiplying CCD camera we used for these bioluminescence assays allows the quantification of biological rhythms that were not detectable using other approaches (Crosby et al, 2017).
Although several mechanisms for circadian regulation of translation have been described (Jouffe et al, 2013; Lipton et al, 2015), we did not find any contribution of rhythmic translation to CRY‐independent rhythms. In fact, the BMAL1‐S6K1 interaction that mediates BMAL1’s interaction with the translational apparatus is absent from CKO cells (Fig EV4C), implying a possible role for CRY proteins in this complex. Instead, we found an overt circadian regulation of PER2::LUC stability that persists in the absence of CRY proteins and which was sufficient to account for the observed PER2::LUC rhythms in a simple mathematical model. Persistent post‐translational regulation of PER stability/activity may also account for the results of earlier overexpression studies, in mammalian cells and flies, where constitutive Per mRNA expression resulted in rhythmic PER protein abundance (Yang & Sehgal, 2001; Yamamoto et al, 2005; Fujimoto et al, 2006), whereas Per overexpression should really abolish rhythms if Per mRNA levels are the fundamental state variable of the oscillation. This interpretation has marked similarities with recent reports in the fungal clock model, Neurospora crassa, where experiments have suggested that post‐translationally regulated cycles in the activity of the FRQ clock protein, not its turnover, are the critical determinant of downstream circadian gene regulation (Larrondo et al, 2015).
Indeed, our observations may not be particularly surprising when one considers that post‐translational regulation of circadian timekeeping is ubiquitous in eukaryotes, with the period‐determining function of CK1δ/ε and GSK3α/β being conserved between the animal, plant and fungal clocks (Lee et al, 2009; Hirota et al, 2010; O’Neill et al, 2011; Yao & Shafer, 2014; Causton et al, 2015; Wong & O’Neill, 2018), despite their clock protein targets being highly dissimilar between phylogenetic kingdoms. Importantly, we observed that pharmacological inhibition of these kinases elicited the same period‐lengthening and period‐shortening effects on CRY‐independent rhythms as on WT rhythms. This has implications for our understanding of the role that these kinases play in the cellular clock mechanism, since in the absence of TTFL‐mediated timekeeping their effects cannot be executed through regulation of any known transcriptional clock component.
Given similar findings across a range of model systems, including isolated red blood cells (Wong & O’Neill, 2018), the simplest interpretation of our findings entails an underlying, evolutionarily conserved post‐translational timekeeping mechanism: a “cytoscillator” (Hastings et al, 2008) that involves CK1δ/ε and GSK3α/β, and can function independently of canonical clock proteins, but normally reciprocally regulates with cycles of clock protein activity through changes in gene expression (Qin et al, 2015). This cytoscillator confers 24‐h periodicity upon the activity and stability of PER2, and most likely to other clock protein transcription factors as well (Fig 3D). However, a purely post‐translational timing mechanism should be rather sensitive to environmental perturbations and biological noise (Ladbury & Arold, 2012), as seen for CKO cells. Due to the geometric nature of their underlying oscillatory mechanism, relaxation oscillators are known to be particularly insensitive to external perturbations and are prevalent in noisy biological systems (Muratov & Vanden‐Eijnden, 2008). We therefore suggest that in WT cells, low amplitude, cytoscillator‐driven circadian cycles of clock protein activity are coupled with, reinforced and amplified by a damped TTFL‐based relaxation oscillation of stochastic frequency (Chickarmane et al, 2007), resulting in high‐amplitude, sustained circadian rhythms in both clock and clock‐controlled gene expression. Indeed, mathematical modelling shows that such coupling can both drive the emergence of sustained oscillations in overdamped systems (In et al, 2003) and play an important role in maintaining robust oscillations in a random environment (Medvedev, 2010). This model is consistent with recent observations in the clocks of the prokaryotic cyanobacterium Synechoccocus elongatus (Qin et al, 2010; Teng et al, 2013) as well as the fungus Neurospora crassa (Larrondo et al, 2015), and the alga Ostreococcus tauri (Feeney et al, 2016b; see Appendix for an extended discussion).
This model is attractive for several reasons. First, it may explain the discrepancy between SCN and behavioural studies in CKO mice, in that residual timekeeping can be observed in cultured SCN PER2::LUC activity, whereas behavioural rhythmicity is not observed in constant darkness following standard 12h:12h light:dark entrainment, but is expressed under specific non‐standard conditions (Iijima et al, 2005; Ono et al, 2013b). Considering the CKO cellular clock’s shorter intrinsic period, as well as the profound robustness conferred upon SCN timekeeping by interneuronal coupling (Yamaguchi et al, 2003; Welsh et al, 2010;), it seems plausible that 24‐h cycles may simply lie outside the range of circadian entrainment for CKO SCN in vivo, similar to the tau mutant hamster and humans with familial advanced sleep phase syndrome (Ptáček et al, 2007; Meng et al, 2008). Whereas ex vivo, or following the strong synchronising cue imposed by transition from constant light to constant darkness (Chen et al, 2008), non‐transcriptional cellular mechanisms are sufficient to impart circadian regulation to CKO neuronal activity that is amplified by neuropeptidergic Ca2+/cAMP‐signalling (O’Neill & Reddy, 2012), facilitating the same temporal consolidation of locomotor activity observed in wild‐type mice but with shorter period and less precision.
Second, whilst the evidence is indisputable that transcriptional feedback repression is critical for circadian co‐ordination of global gene expression, physiology and behaviour, the evidence that these regulatory gene expression circuits are inherently possessed of approximately 24‐h rhythmicity is weak (reviewed in Lakin‐Thomas, 2006; Putker & O’Neill, 2016; Wong & O’Neill, 2018). Post‐translational regulation of clock protein stability, activity and localisation, however, is already well established as the primary determinant of the delay constants that allow the oscillation to persist with a period of about 1 day in all studied eukaryotic cells (Gallego & Virshup, 2007; van Ooijen et al, 2011; Top et al, 2018; Wong & O’Neill, 2018). We simply suggest that transcriptional feedback repression is not essential for circadian timekeeping per se, but amplifies the rhythms to increase robustness via hysteresis, when engaged, and also to confer tissue and cell‐type‐specific functionality (Wong & O’Neill, 2018). Our paradigm here being the cell division cycle, where the essential timing mechanism is also post‐translational, and persists in enucleated cells (Hara et al, 1980; Pomerening et al, 2005).
Third, there is no evidence that TTFL‐mediated oscillations would not damp to a steady state without post‐translational input (Wong & O’Neill, 2018). In contrast, there are several examples in the eukaryotic lineage, where circadian timekeeping persists in the absence of cycling gene expression (Lakin‐Thomas, 2006; Sweeney & Haxo, 1961; O’Neill & Reddy, 2011; O’Neill et al, 2011). For example, the period of circadian rhythms in human cells and Ostreococcus tauri is regulated by CK1, both in the presence and absence of nascent transcription (O’Neill et al, 2011; Beale et al, 2019) similar to the rhythm reported by PER2::LUC in CKO cells we report here.
Interestingly, the concept of the eukaryotic post‐translational clock mechanism we propose is not new (Roenneberg & Merrow, 1998; Merrow et al, 2006; Qin et al, 2010; Jolley et al, 2012) and resembles the KaiA/B/C mechanism elucidated in cyanobacteria (Nakajima et al, 2005; Teng et al, 2013). The challenge will now be to identify additional factors that, in concert with CK1 and GSK3, and protein phosphatase 1 (Lee et al, 2011), serve as the functional equivalents of KaiA/B/C, allowing reconstitution of the mammalian circadian clock in vitro (Nakajima et al, 2005; Millius et al, 2019).
Here we have uncovered PER2 as a node of interaction between a putative cytoscillator mechanism and the canonical circadian TTFL (Fig 5D). It is unlikely however that PER2 is the only interaction between the two, as Per2 −/− knockout cells and mice exhibit competent circadian timekeeping (Xu et al, 2007), suggesting redundancy in this respect. Indeed, the residual noisy but rhythmic activity of the Nr1d1‐promoter in the absence of both PER1/2 and CRY1/2 (Fig 3D) suggests another point of connection between the cytoscillator and TTFL. Moreover, both CK1 and GSK have been implicated in the phosphorylation and regulation of many other clock proteins (see Table S2 in Causton et al, 2015, also reviewed in O’Neill et al, 2013). Some or all of these targets may play a role in coupling the cytoscillator with TTFL‐mediated clock output. We believe that it is now imperative to delineate the specific means by which the TTFL couples with the cytoscillator to effect changes in circadian phase in order that the two resonate with a common frequency.
Conclusion
Whilst the contribution of clock protein transcription factors to the temporal co‐ordination of gene expression, physiology and behaviour is unambiguous, the primacy of transcriptional feedback repression as the ultimate arbiter of circadian periodicity within eukaryotic cells is not. Similar to the conserved kinase‐dependent regulation of the cell division cycle, we suggest the circadian cycle in diverse eukaryotes is conserved from a common ancestor, with diverse TTFL components having been recruited throughout speciation to impart robustness, signal amplification and functional specificity to the oscillation.
Materials and Methods
Reagents were obtained from Sigma unless stated otherwise. More detailed Materials and Methods can be found in the Appendix.
Mouse work
All animal work was licensed under the UK Animals (Scientific Procedures) Act 1986, with Local Ethical Review by the Medical Research Council. Cry1/2‐null mice were kindly provided by G. T. van der Horst (Erasmus MC, Rotterdam, The Netherlands) (Horst & Muijtjens, 1999), PER2::LUC mice by J. S. Takahashi (UT Southwestern, USA) (Yoo et al, 2004) and Cry1:LUC mice by M. Hastings (MRC LMB, Cambridge, UK) (Maywood et al, 2013). All lines were maintained on a C57BL/6J background. For mouse behavioural studies, two independent recordings were made from male and female CKO PER2::LUC aged 2–5 months, with age‐ and gender‐matched PER2::LUC controls, singly housed in running wheel cages with circadian cabinets (Actimetrics). They were then subject to 7 days 12 h:12 h LD cycles or 7 days constant light (400 lux), and then maintained in constant darkness with weekly water and food changes. Locomotor activity was recorded using running wheel activity and passive infrared detection, which was analysed using the periodogram function of ClockLab (Actimetrics) with a significance threshold of P = 0.0001. SCN organotypic slices from 7‐ to 10‐day‐old pups were prepared as previously described (Hastings et al, 2005), and bioluminescence recorded using photomultiplier tubes (Hamamatsu).
Mammalian cell culture
Primary fibroblasts were isolated from lung tissue (Seluanov et al, 2010) of adult wild‐type (WT) and Cry1 −/−,Cry2 −/− (CKO) PER2::LUC male and female mice, and WT and CKO Cry1:LUC mice. Stable WT, CKO and Cry1 −/−,Cry2 −/−, Per1 −/−, Per2 −/− (CPKO) mouse embryonic fibroblasts (MEFs) expressing transcriptional luciferase reporters for clock gene activity were generated by puromycin selection and cultured as described previously (Valekunja et al, 2013). MEFs were seeded into 96‐well white plates at 104 cells/well and grown to confluency for 5 days under temperature cycles (12 h:12 h, 32°C:37°C) to synchronise circadian rhythms. Primary fibroblasts were cultured as described previously (O’Neill & Hastings, 2008) and immortalised by serial passage (Xu, 2005). CRY deficiency was confirmed by PCR (see Appendix) and Western blotting [guinea pig‐anti‐CRY1 and CRY2 antibodies (Lamia et al, 2011)]. NIH3T3 fibroblasts expressing SV40::LUC have been described before (Feeney et al, 2016a).
Luciferase recordings
Fibroblast recordings were performed in air medium (either HEPES or MOPS buffered (20 mM), either in airtight sealed dishes (in non‐humidified conditions) or open in humidified conditions (0% CO2). Air medium stock was prepared as described previously (O’Neill & Hastings, 2008) and supplemented with 2% B‐27 (Life Technologies, 50×), 1 mM luciferin (Biosynth AG), 1× glutamax (Life Technologies), 100 units/ml penicillin/100 µg/ml streptomycin and 1% FetalClone™ III serum (HyClone™). Final osmolarity was adjusted to 350 mOsm with NaCl. Recordings were preceded by appropriate synchronisation (see Appendix for details) in the presence of 0.3 mM luciferin to prevent artificially high bioluminescence activity at the start of the recording, and started immediately after a medium change from culture medium into air medium. The presented MEF recordings were performed in an ALLIGATOR (Crosby et al, 2017) and employed bicarbonate‐buffered Dulbecco’s modified Eagle medium (10569010) with penicillin/streptomycin and 1 mM luciferin in a humidified incubator at 5% CO2, also supplemented with 2% B‐27 and 10% FetalClone™ III serum. A range of other media conditions were explored but did not produce detectable bioluminescence rhythms in CKO or CPKO cells (not shown). For pharmacological perturbation experiments (unless stated otherwise in the text), cells were changed into drug‐containing air medium from the start of the recording. Mock treatments were carried out with DMSO or ethanol as appropriate.
Bioluminescence recordings were performed in a lumicycle (Actimetrics), a LB962 plate reader (Berthold technologies) or an ALLIGATOR (Cairn Research). Acute luciferase assays were performed using a Spark 10 M microplate reader (Tecan).
Biochemistry
The number of PER2 molecules was determined by harvesting a known number of synchronised WT and CKO cells at the peak of PER2 expression and comparing the Luciferase activity to a standard curve of recombinant luciferase (see Appendix for details). Three technical replicates were measured in every experiment, and the experiment was carried out three times. A representative experiment is shown.
For determining Per2::Luc and Bmal1 mRNA levels, synchronised cells were harvested from constant conditions in triplicate every 4 h from 24 h up to 48 h after media change. RNA extraction and qPCR were performed as detailed in the Appendix. Analysis involved three technical and three biological replicates. Relative amounts of mRNA were determined by comparing the samples to a standard curve and expressed relatively to ribosomal RNA Rns18s.
For comparing longitudinal PER2::LUC recordings to the actual PER2::LUC protein levels (longitudinal vs. acute luciferase assays), synchronised WT and CKO cells (cultured in absence of luciferin) were harvested every hour (in triplicate) from 16 h up to 64 h after media change, whilst co‐cultures were recorded for bioluminescence in the presence of luciferin. Luciferase activity in acute assays was determined as detailed in Appendix.
For assaying the interaction between BMAL1 and PER2::LUC, synchronised cells were harvested directly from temperature cycles at the expected peak of PER2::LUC expression (4 h after change to 32°C) and BMAL1 was precipitated as described in Appendix. PER2::LUC co‐immunoprecipitation was measured in a luciferase assay by mixing the BMAL1‐loaded beads in luciferase assay buffer (15 mM MgSO4, 30 mM HEPES, 300 µM luciferin, 1 mM ATP, 10 mM 2‐mercaptoethanol) and measuring luciferase activity in a Berthold plate reader. The results were corrected for input and plotted relatively to the WT IgG pulldown.
To study the interaction of BMAL1 with S6K and eIF4, cells were synchronised by a 2‐h dexamethasone pulse, after which they were changed into normal growth medium. 12 and 24 h after the medium change, BMAL1 immunoprecipitation was executed as described in Appendix. Samples were analysed by Western blot for the presence of BMAL1, S6K and eIF4 (Cell Signaling, resp. #2708 and #2013).
Drosophila experiments
All fly strains were kept in standard cornmeal food under 12 h:12 h LD cycles at constant 25°C (LD cycles). The following control strains were included in the experiments: per01, Canton S and w1118. The generation of TimOut flies, crossings with XLG‐luc flies (Veleri et al, 2003) and details of recordings are described in Appendix. In short, 3‐ to 7‐day‐old flies were entrained for 3 days LD cycles before being loaded individually into the wells of a microtiter plate containing the food‐luciferin substrate (15 mM luciferin). Recordings were performed under constant darkness at 26°C over 7 days. Bioluminescence from each fly was background subtracted, summed into 2‐h bins and then detrended using a 24‐h moving average. Rhythmicity of averaged traces was tested by least‐square fitting, comparing a circadian damped sine wave with the null hypothesis (straight line), as described below.
Analysis
All analyses were performed in GraphPad Prism versions 7 and 8. Where indicated, data were detrended using moving average subtraction, where temporal window of the moving average was refined iteratively until it matched with the period of oscillation derived as follows. Period analysis was performed either manually, or by least‐square fitting to a circadian damped sine wave with a linear baseline:
where m is the gradient of the baseline, c is the y offset, k describes the rate of dampening, a the amplitude, r the phase and p the period. Reported P‐values for the curve fit are those produced by the comparison of fits function in Prism 8, where the null hypothesis was a straight line (y = mx + c), i.e. change over time but with no oscillatory component. The simpler model was preferred unless the sine wave fit produced a better fit with P < 0.05.
For the mathematical model in 4E, we assumed that PER2::LUC translation at time (t) is a function of Per2::Luc mRNA abundance, corrected for the changes we observed for global translation rate over time; and that PER2::LUC degradation rate follows one‐phase exponential decay kinetics where the decay constant is defined by a sine wave with 24‐h periodicity, with the amplitude, phase and other parameters being derived entirely from experimental measurements. See Appendix for details.
Author contributions
MP and JSON designed the study, analysed the data and wrote the manuscript. AZ and NMR performed mouse behavioural studies. MP, DCSW, ES, NPH, KAF, MVO, RSE and JSON performed cell experiments. CPS and AS generated MEF cell lines. MDE performed SCN experiments. K‐FC, RF, NP and JSON performed fly experiments. JEC performed tissue collection and husbandry. All authors commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Acknowledgements
We thank biomedical technical staff at Medical Research Council (MRC) Ares facility and LMB facilities for assistance, G.T. van der Horst and J.S. Takahashi for sharing rodent models, M.H. Hastings and E.S. Maywood for providing reagents and input, K. Lamia for providing reagents, and P. Crosby, D.S. Tourigny, J.E.C. Jepson, C.P. Kyriacou and H.R. Pelham for valuable discussion. MP was supported by the Dutch Cancer Foundation (KWF, BUIT‐2014‐6637) and EMBO (ALTF‐654‐2014). JON was supported by the Medical Research Council (MC_UP_1201/4) and the Wellcome Trust (093734/Z/10/Z). NP and RF were supported by the Deutsche Forschungsgemeinschaft FKZ (Pe1798/2‐1). AS and CPS were supported by the National Institutes of Health (GM118102). NMR was supported by the Medical Research Council (MR/S022023/1).
The EMBO Journal (2021) 40: e106745.
Data availability
This study includes no data deposited in external repositories.
References
- Anand SN, Maywood ES, Chesham JE, Joynson G, Banks GT, Hastings MH, Nolan PM (2013) Distinct and separable roles for endogenous CRY1 and CRY2 within the circadian molecular clockwork of the suprachiasmatic nucleus, as revealed by the Fbxl3(Afh) mutation. J Neurosci 33: 7145–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L et al (2007) An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free‐running and entrained conditions. J Pharmacol Exp Ther 322: 730–738 [DOI] [PubMed] [Google Scholar]
- Beale AD, Kruchek E, Kitcatt SJ, Henslee EA, Parry JSW, Braun G, Jabr R, von Schantz M, O’Neill JS, Labeed FH (2019) Casein kinase 1 underlies temperature compensation of circadian rhythms in human red blood cells. J Biol Rhythms 34: 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton HC, Feeney KA, Ziegler CA, O’Neill JS (2015) Metabolic cycles in yeast share features conserved among circadian rhythms. Curr Biol 25: 1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Seo DO, Bell E, Von Gall C, Lee DC (2008) Strong resetting of the mammalian clock by constant light followed by constant darkness. J Neurosci 28: 11839–11847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo S‐HH, Takahashi JS, Lee C (2009) Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell 36: 417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickarmane V, Kholodenko BN, Sauro HM (2007) Oscillatory dynamics arising from competitive inhibition and multisite phosphorylation. J Theor Biol 244: 68–76 [DOI] [PubMed] [Google Scholar]
- Chiou YY, Yang Y, Rashid N, Ye R, Selby CP, Sancar A (2016) Mammalian period represses and de‐represses transcription by displacing CLOCK‐BMAL1 from promoters in a cryptochrome‐dependent manner. Proc Natl Acad Sci USA 113: E6072–E6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C‐S, Yoon HJ, Kim JY, Woo HA, Rhee SG (2014) Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc Natl Acad Sci USA 111: 12043–12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby P, Hoyle NP, O’Neill JS (2017) Flexible measurement of bioluminescent reporters using an automated longitudinal luciferase imaging gas‐ and temperature‐optimized recorder (ALLIGATOR). J Vis Exp 130: 56623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA et al (2019) Insulin/IGF‐1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177: 896–909.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM (2007) Peripheral circadian oscillators require CLOCK. Curr Biol 17: 538–539 [DOI] [PubMed] [Google Scholar]
- Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney K et al (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485: 459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MD, Brancaccio M, Chesham JE, Maywood ES, Hastings MH (2016) Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc Natl Acad Sci USA 113: 2732–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Vielhaber EL, Hinz WA, Virshup DM (2002) The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iε. J Biol Chem 277: 17248–17254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Pan H, Liu AC, Welsh DK (2012) Cry1‐/‐ circadian rhythmicity depends on SCN intercellular coupling. J Biol Rhythms 27: 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Hida A, Anderson DA, Izumo M, Johnson CH (2007) Cycling of CRYPTOCHROME proteins is not necessary for circadian‐clock function in mammalian fibroblasts. Curr Biol 17: 1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney KA, Putker M, Brancaccio M, O’Neill JS (2016a) In‐depth characterization of firefly luciferase as a reporter of circadian gene expression in mammalian cells. J Biol Rhythms 31: 540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney KAKA, Hansen LLL, Putker M, Olivares‐Yañez C, Day J, Eades LJLJ, Larrondo LFLF, Hoyle NPNP, O’Neill JS, van Ooijen G et al (2016b) Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532: 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Yagita K, Okamura H (2006) Does mPER2 protein oscillate without its coding mRNA cycling?: post‐transcriptional regulation by cell clock. Genes Cells 11: 525–530 [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM (2007) Post‐translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148 [DOI] [PubMed] [Google Scholar]
- Hara K, Tydeman P, Kirschner M (1980) A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. PNAS 77: 462–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, McMahon DG, Maywood ES (2005) Analysis of circadian mechanisms in the suprachiasmatic nucleus by transgenesis and biolistic transfection. Methods Enzymol 393: 579–592 [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, O’Neill JS (2008) Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol 18: R805–R815 [DOI] [PubMed] [Google Scholar]
- Hirota T, Lewis WG, Liu AC, Wook J, Schultz PG, Kay SA (2008) A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK‐3. Proc Natl Acad Sci USA 105: 20746–20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, Garcia M, Peters EC, Etchegaray JP, Traver D et al (2010) High‐throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIα as a clock regulatory kinase. PLoS Biol 8: e1000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lee JW, John PCS, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA et al (2012) Identification of small molecule activators of cryptochrome. Science 337: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, Bray LK, Thomas JM, Dunn K, Blaikley J et al (2017) Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci Transl Med 9: eaal2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Yamaguchi S, van der Horst GTJ, Bonnefont X, Okamura H, Shibata S (2005) Altered food‐anticipatory activity rhythm in Cryptochrome‐deficient mice. Neurosci Res 52: 166–173 [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N (2005) A role for glycogen synthase kinase‐3beta in the mammalian circadian clock. J Biol Chem 280: 29397–29402 [DOI] [PubMed] [Google Scholar]
- In V, Bulsara AR, Palacios A, Longhini P, Kho A, Neff JD (2003) Coupling‐induced oscillations in overdamped bistable systems. Phys Rev E 68: 4–7 [DOI] [PubMed] [Google Scholar]
- Jiang S, Zhang M, Sun J, Yang X (2018) Casein kinase 1α: biological mechanisms and theranostic potential. Cell Commun Signal 16: 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley CC, Ode KL, Ueda HR (2012) A design principle for a posttranslational biochemical oscillator. Cell Rep 2: 938–950 [DOI] [PubMed] [Google Scholar]
- Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, Gachon F (2013) The circadian clock coordinates ribosome biogenesis. PLoS Biol 11: e1001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205 [DOI] [PubMed] [Google Scholar]
- Ladbury JE, Arold ST (2012) Noise in cellular signaling pathways: causes and effects. Trends Biochem Sci 37: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin‐Thomas PL (2006) Transcriptional feedback oscillators: maybe, maybe not. J Biol Rhythms 21: 83–92 [DOI] [PubMed] [Google Scholar]
- Lamaze A, Öztürk‐Çolak A, Fischer R, Peschel N, Koh K, Jepson JE (2017) Regulation of sleep plasticity by a thermo‐sensitive circuit in Drosophila. Sci Rep 7: 40304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480: 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Wang LL, Diemer T, Welsh DK (2016) NPAS2 compensates for loss of CLOCK in peripheral circadian oscillators. PLoS Genet 12: e1005882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrondo LF, Olivares‐Yanez C, Baker CL, Loros JJ, Dunlap JC (2015) Decoupling circadian clock protein turnover from circadian period determination. Science 347: 1257277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen R, Lee Y, Yoo S, Lee C (2009) Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc Natl Acad Sci USA 106: 21359–21364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen R, Kim H, Etchegaray J‐P, Weaver DR, Lee C (2011) The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA 108: 16451–16456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH, Chuang DM (2006) Differential roles of glycogen synthase kinase‐3 isoforms in the regulation of transcriptional activation. J Biol Chem 281: 30479–30484 [DOI] [PubMed] [Google Scholar]
- Lipton JO, Yuan ED, Boyle LM, Ebrahimi‐Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M (2015) The circadian protein BMAL1 regulates translation in response to S6K1‐mediated phosphorylation. Cell 161: 1138–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM et al (2007) Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129: 605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA (2008) Redundant function of REV‐ERBalpha and beta and non‐essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet 4: e1000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH, Brien JAO, Hastings MH (2011) A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA 108: 14306–14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Drynan L, Chesham JE, Edwards MD, Dardente H, Fustin J‐M, Hazlerigg DG, O’Neill JS, Codner GF, Smyllie NJ et al (2013) Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1‐luc transgenic reporter mouse. Proc Natl Acad Sci USA 110: 9547–9552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medunjanin S, Schleithoff L, Fiegehenn C, Weinert S, Zuschratter W, Braun‐Dullaeus RC (2016) GSK‐3β controls NF‐kappaB activity via IKKγ/NEMO. Sci Rep 6: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev GS (2010) Synchronization of coupled stochastic limit cycle oscillators. Phys Lett A 374: 1712–1720 [Google Scholar]
- Meng Q‐JJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NRJ, Piggins HD et al (2008) Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam‐Webster Dictionary (2020) ‘Robust’.
- Merrow M, Mazzotta G, Chen Z, Roenneberg T (2006) The right place at the right time: regulation of daily timing by phosphorylation. Genes Dev 20: 2629–2633 [DOI] [PubMed] [Google Scholar]
- Millius A, Ode KL, Ueda HR (2019) A period without PER: understanding 24‐hour rhythms without classic transcription and translation feedback loops [version 1; peer review: 2 approved]. F1000 Res 8: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratov CB, Vanden‐Eijnden E (2008) Noise‐induced mixed‐mode oscillations in a relaxation oscillator near the onset of a limit cycle. Chaos 18: 015111 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T (2005) Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro . Science 308: 414–415 [DOI] [PubMed] [Google Scholar]
- Nangle SN, Rosensweig C, Koike N, Tei H, Takahashi JS, Carla B (2014) Molecular assembly of the period‐cryptochrome circadian transcriptional repressor complex. Elife 3: e03674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimamurthy R, Hunt SR, Lu Y, Fustin J, Okamura H, Partch CL, Forger DB, Kim JK, Virshup DM (2018) CK1 δ / e protein kinase primes the PER2 circadian phosphoswitch. Proc Natl Acad Sci USA 115: 5986–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Hastings MH (2008) Increased coherence of circadian rhythms in mature fibroblast cultures. J Biol Rhythms 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Reddy AB (2011) Circadian clocks in human red blood cells. Nature 469: 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Van OG, Dixon LE, Troein C, Corellou F, Bouget F‐Y, Reddy AB, Millar AJ (2011) Circadian rhythms persist without transcription in a eukaryote. Nature 469: 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Reddy AB (2012) The essential role of cAMP/Ca2+ signalling in mammalian circadian timekeeping. Biochem Soc Trans 40: 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Hastings MH (2013) Cellular mechanisms of circadian pacemaking: beyond transcriptional loops. Handb Exp Pharmacol 217: 67–103 [DOI] [PubMed] [Google Scholar]
- Ode KL, Ukai H, Susaki EA, Narumi R, Matsumoto K, Hara J, Koide N, Abe T, Kanemaki MT, Kiyonari H et al (2017) Knockout‐rescue embryonic stem cell‐derived mouse reveals circadian‐period control by quality and quantity of CRY1. Mol Cell 65: 176–190 [DOI] [PubMed] [Google Scholar]
- Ono D, Honma S, Honma K‐I (2013a) Postnatal constant light compensates Cryptochrome1 and 2 double deficiency for disruption of circadian behavioral rhythms in mice under constant dark. PLoS One 8: e80615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono D, Honma S, Honma K (2013b) Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nat Commun 4: 1666 [DOI] [PubMed] [Google Scholar]
- Philpott JM, Narasimamurthy R, Ricci CG, Freeberg AM, Hunt SR, Yee LE, Pelofsky RS, Tripathi S, Virshup DM, Partch CL (2020) Casein kinase 1 dynamics underlie substrate selectivity and the PER2 circadian phosphoswitch. Elife 9: 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25: 159–184 [DOI] [PubMed] [Google Scholar]
- Pomerening JR, Sun YK, Ferrell JE (2005) Systems‐level dissection of the cell‐cycle oscillator: bypassing positive feedback produces damped oscillations. Cell 122: 565–578 [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Luis‐Lopez‐Molina ZJ, Duboule D, Albrecht U, Schibler U (2002) The orphan nuclear receptor REV‐ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260 [DOI] [PubMed] [Google Scholar]
- Ptáček LJ, Jones CR, Fu YH (2007) Novel insights from genetic and molecular characterization of the human clock. Cold Spring Harb Symp Quant Biol 72: 273–277 [DOI] [PubMed] [Google Scholar]
- Putker M, O’Neill JS (2016) Reciprocal control of the circadian clock and cellular redox state ‐ a critical appraisal. Mol Cells 39: 6–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Byrne M, Xu Y, Mori T, Johnson CH (2010) Coupling of a core post‐translational pacemaker to a slave transcription/translation feedback loop in a circadian system. PLoS Biol 8: e1000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Mori T, Zhang Y, Johnson CH (2015) PER2 differentially regulates clock phosphorylation versus transcription by reciprocal switching of CK1 activity. J Biol Rhythms 30: 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A (2011) SCF/bTrCP promotes glycogen synthase kinase 3‐dependent degradation of the Nrf2 transcription factor in a Keap1‐independent manner. Mol Cell Biol 31: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Valekunja UK, Stangherlin A, Howell SA, Snijders AP, Damodaran G, Reddy AB (2020) Circadian rhythms in the absence of the clock gene Bmal1. Science 806: 800–806 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418: 935–941 [DOI] [PubMed] [Google Scholar]
- Robertson H, Hayes JD, Sutherland C (2018) A partnership with the proteasome; the destructive nature of GSK3. Biochem Pharmacol 147: 77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M (1998) Molecular circadian oscillators: an alternative hypothesis. J Biol Rhythm 13: 167–179 [DOI] [PubMed] [Google Scholar]
- Rosbash M (2009) The implications of multiple circadian clock origins. PLoS Biol 7: 0421–0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone‐Corsi P (2010) Regulation of BMAL1 protein stability and circadian function by GSK3β‐mediated phosphorylation. PLoS One 5: e8561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Yamada RG, Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB (2006) Feedback repression is required for mammalian circadian clock function. Nat Genet 38: 312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Price JL, Man B, Young MW (1994) Loss of circadian Behavioral Rhythms and per RNA oscillations in the Drosophila mutant timeless. Science 263: 1603–1606 [DOI] [PubMed] [Google Scholar]
- Sehgal A, Rothenfluh‐Hilfiker A, Hunter‐Ensor M, Chen Y, Myers MP, Young MW (1995) Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 270: 808–810 [DOI] [PubMed] [Google Scholar]
- Seluanov A, Vaidya A, Gorbunova V (2010) Establishing primary adult fibroblast cultures from rodents. J Vis Exp 44: 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch K‐F, Weitz CJ (2009) Daily rhythms of food‐anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA 106: 6808–6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney B, Haxo F (1961) Persistence of a photosynthetic rhythm in enucleated acetabularia. Science 134: 1361–1363 [DOI] [PubMed] [Google Scholar]
- Takahashi JS (2016) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18: 164–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S‐W, Mukherji S, Moffitt JR, de Buyl S, O'Shea EK (2013) Robust circadian oscillations in growing cyanobacteria require transcriptional feedback. Science 340: 737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS et al (1998) Role of mouse cryptochrome blue‐light photoreceptor in circadian photoresponses. Science 282: 1490–1494 [DOI] [PubMed] [Google Scholar]
- Tokuda IT, Ono D, Ananthasubramaniam B, Honma S, Honma K‐I, Herzel H (2015) Coupling controls the synchrony of clock cells in development and knockouts. Biophys J 109: 2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita J, Nakajima M, Kondo T, Iwasaki H (2005) No transcription‐translation feedback in circadian rhythm of KaiC phosphorylation. Science 307: 251–254 [DOI] [PubMed] [Google Scholar]
- Top D, O’neil JL, Merz GE, Dusad K, Crane BR, Young MW(2018) CK1/doubletime activity delays transcription activation in the circadian clock. Elife 7: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullai JW, Tacheva S, Owens LJ, Graham JR, Cooper GM (2011) AP‐1 is a component of the transcriptional network regulated by GSK‐3 in quiescent cells. PLoS One 6: e20150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR (2007) Systems biology of mammalian circadian clocks. Cold Spring Harb Symp Quant Biol 72: 365–380 [DOI] [PubMed] [Google Scholar]
- Ukai‐Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR (2011) Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell 144: 268–281 [DOI] [PubMed] [Google Scholar]
- Valekunja UK, Edgar RS, Oklejewicz M, Van Der Horst GTJ, O’Neill JS, Tamanini F, Turner DJ, Reddy AB (2013) Histone methyltransferase MLL3 contributes to genome‐scale circadian transcription. Proc Natl Acad Sci USA 110: 1554–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Horst GTJ, Muijtjens M (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 3495: 627–630 [DOI] [PubMed] [Google Scholar]
- van Ooijen G, Dixon LE, Troein C, Millar AJ (2011) Proteasome function is required for biological timing throughout the twenty‐four hour cycle. Curr Biol 21: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleri S, Brandes C, Helfrich‐Förster C, Hall JC, Stanewsky R (2003) A self‐sustaining, light‐entrainable circadian oscillator in the Drosophila brain. Curr Biol 13: 1758–1767 [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J et al (1999) Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA 96: 12114–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72: 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR (2018) NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus . Elife 3: 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DC, O’Neill JS (2018) Non‐transcriptional processes in circadian rhythm generation. Curr Opin Physiol 5: 117–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J (2005) Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr Protoc Mol Biol Chapter 28: 1–8 [DOI] [PubMed] [Google Scholar]
- Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptacek LJ (2007) Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H (2003) Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302: 1408–1412 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yagita K, Okamura H (2005) Role of cyclic mPer2 expression in the mammalian cellular clock. Mol Cell Biol 25: 1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sehgal A (2001) Role of molecular oscillations in generating behavioral rhythms in Drosophila . Neuron 29: 453–467 [DOI] [PubMed] [Google Scholar]
- Yang G, Wright CJ, Hinson MD, Fernando AP, Sengupta S, Biswas C, La P, Dennery PA (2014) Oxidative stress and inflammation modulate Rev‐erbα signaling in the neonatal lung and affect circadian rhythmicity. Antioxid Redox Signal 21: 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Shafer OT (2014) The Drosophila circadian clock is a variable coupled network of multiple peptidergic units. Science 343: 1516–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Selby CP, Chiou Y‐Y, Ozkan‐Dagliyan I, Gaddameedhi S, Sancar A (2014) Dual modes of CLOCK:BMAL1 inhibition mediated by Cryptochrome and Period proteins in the mammalian circadian clock. Genes Dev 28: 1989–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S‐H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H‐K, Oh WJ, Yoo OJ et al (2004) PERIOD2:LUCIFERASE real‐time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View
Review Process File
Data Availability Statement
This study includes no data deposited in external repositories.


