Figure 2.
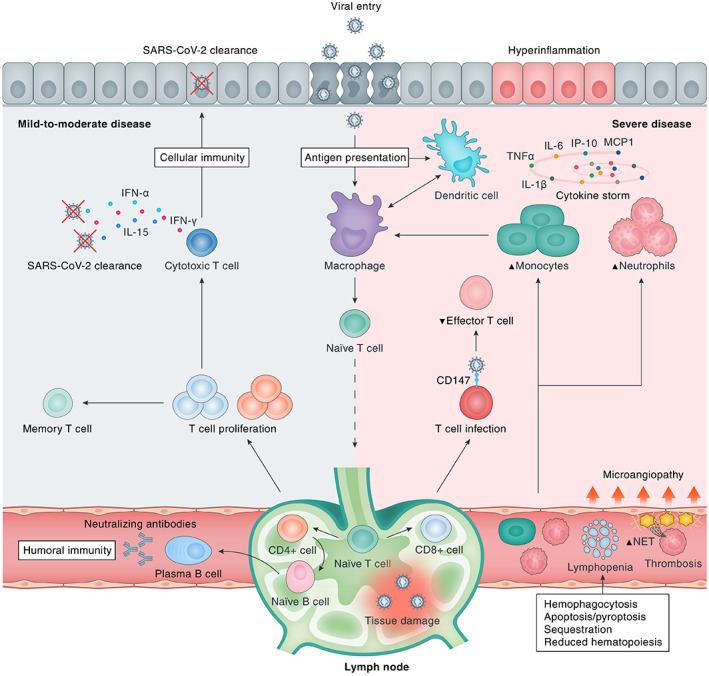
Immunological response to SARS‐CoV‐2 infection. Upon viral cell entry, SARS‐CoV‐2 antigens are processed by the innate immune system through antigen‐presenting cells (APCs), e.g. epithelial cells, macrophages, and/or dendritic cells. Subsequently, the adaptive immune system is activated by migration of APCs to the lymphoid system. Upon antigen recognition, T‐lymphocytes proliferate and differentiate into CD4+ and CD8+ T‐lymphocytes that are responsible for sequential events including cytokine production, activation of naïve B‐lymphocytes, and clearance of infected cells (CD8+ cytotoxic T‐lymphocytes). B‐lymphocytes proliferate and differentiate into plasma cells that produce large numbers of neutralising antibodies, representing humoral immunity. A bulk of cytokines is induced upon SARS‐CoV‐2 infection, most of which contribute to hyperinflammation as constituents of the ‘cytokine storm’ in severe disease (e.g. IL‐6, TNF‐α, IL‐1β, IP‐10, MCP‐1, CSFs, and IL‐17A), whereas others are particularly important for viral clearance (e.g. IL‐15, IFN‐α, IL‐12, IL‐21, and IFN‐γ) in mild‐to‐moderate disease. Severe COVID‐19 is marked by dysfunction of certain immune cells, with relatively increased abundances of neutrophils and monocytes and decreased levels of effector T‐lymphocytes. In addition, multiple downstream pathophysiological processes are activated, including an increased thrombogenic state [microangiopathy, formation of neutrophil extracellular traps (NETs)], haemophagocytosis, reduced haematopoiesis, and increased apoptosis/pyroptosis. CD147, cluster of differentiation 147; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
