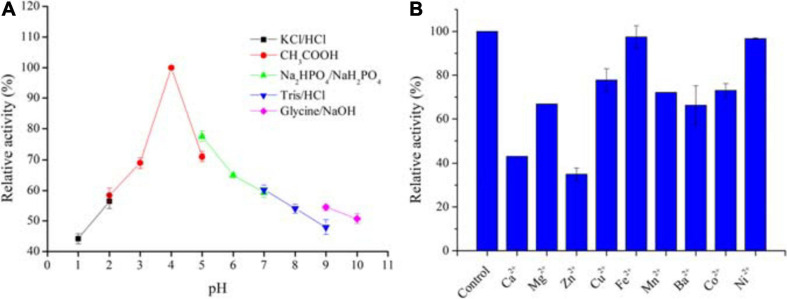
FIGURE 7.
Stabilities of AaSOD in buffers with different pH values (A) and effect of divalent ions on the AaSOD activity (B). The purified enzyme was incubated in the buffer systems of 200 mM KCl–HCl buffer (pH 1.0–2.0), acetate buffer (pH 2.0–5.0), sodium phosphate buffer (pH 5.0–7.0), Tris–HCl buffer (pH 7.0–9.0), and glycine–NaOH buffer (pH 9.0–10.0) for 1 h, 25°C. The maximal activity was taken as 100% for relative activity calculation. For metal ions effects, the purified enzyme was incubated with 1 mM of divalent metal ions in 50 mM Tris–HCl buffer (pH 8.0), at 25°C for 30 min. The mixture with no ions supplementation was used as a control for relative activity calculation.