Figure 5.
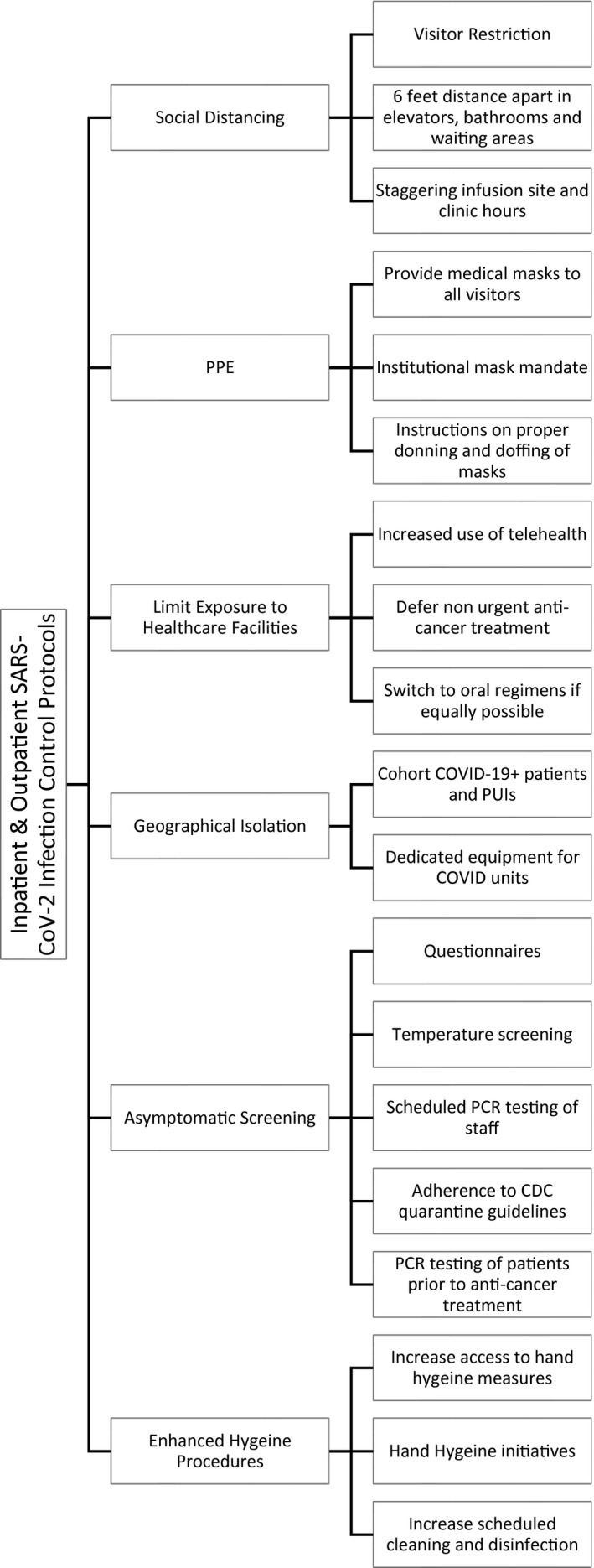
Inpatient and outpatient SARS‐CoV‐2 infection control policies. Inpatient and outpatient SARS‐CoV‐2 infection control policies included the following: 1) visitor restriction (visitors were prohibited from the treatment areas or clinic unless they are needed for patient safety and patient care as preapproved by the nursing manager), 2) defermentof nonurgent anticancer treatment (testing all patients suspected of having COVID and deferring anticancer treatment if possible until test results arrive), 3) an institutional mask mandate (all visitors, adult/pediatric patients, and staff are required to wear a surgical mask upon entry to the facility), 4) staggering infusion site and clinic hours (clinic hours and infusion hours were not held on overlapping times but instead were given dedicated time slots), 5) increased use of telehealth (increased use of telemedicine visits when appropriate; when there is high suspicion for SARS‐CoV‐2 infection, then the patient's appointment is rescheduled or converted to a telemedicine visit as appropriate), 6) scheduled polymerase chain reaction testing of staff (all staff must undergo SARS‐CoV‐2 polymerase chain reaction testing at scheduled intervals and adhere to appropriate CDC guidelines on the basis of test results and symptoms), 7) questionnaires (screening all staff and adult/pediatric patients before they enter inpatient or outpatient facilities for infectious symptoms, travel history, and regular antipyretic use), 8) temperature screening (temperature checks of all staff and adult/pediatric patients before they enter any health care facility), 9) hand hygiene initiatives (encouraging staff to enhance sanitation of high‐traffic areas and practice good hygiene techniques), and 10) increased access to hand hygiene measures (increasing the availability and enforcing the use of hand sanitization dispensers near high‐use areas [elevators, doors, and handles]). CDC indicates Centers for Disease Control and Prevention; COVID, coronavirus disease; COVID‐19, coronavirus disease 2019; PCR, polymerase chain reaction; PPE, personal protective equipment; PUI, person under investigation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
