Figure 8.
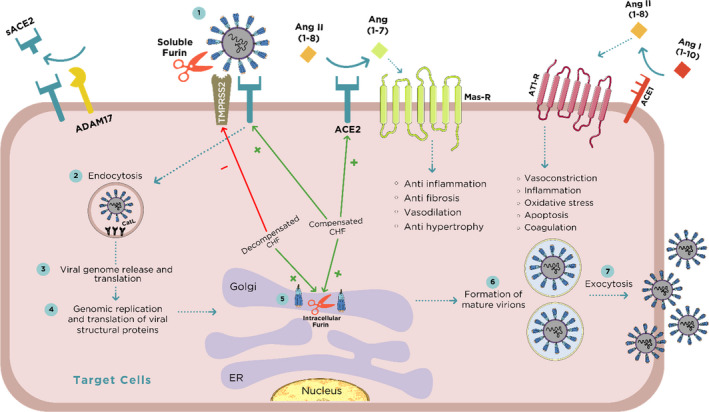
SARS‐CoV‐2 binding, activation, invasion and replication in target cells. The initial step after the invasion of SARS‐CoV‐2 is binding to membranal ACE2 widely expressed in vital organs including lung, heart and kidney. ACE2 is responsible for the conversion of Ang II to Ang 1‐7 which exerts beneficial effects on the cardiac tissue such as vasodilation, anti‐fibrosis and anti‐inflammation via Mas receptor (MasR). The binding of SARS‐CoV‐2 to ACE2 is preceded by TMPRSS2/furin‐mediated exposure of the viral receptor binding protein (RBP) localized to S‐glycoprotein (S1 domain of the viral spike) and revealing the viral effusion site on S2 domain. Furin is expressed in these tissues both intracellularly and in the circulation as a free enzyme, making it a key factor along TMPRSS2 in the uncovering of RBP and eventually in SARS‐CoV‐2 transmission. In addition, furin enhances the affinity of the virus to ACE2, not only by exposing the viral binding site on S1 domain but also by revealing the effusion site on the S2 domain in the viral spike. Consequently, the virus undergoes endocytosis and massive replication accompanied by profound activation by the abundant intracellular furin and Cathepsin L (Cat‐L). The activated intracellular SARS‐CoV‐2 undergoes exocytosis where it binds again to ACE2 elsewhere, thus creating a vicious feed‐forward devastating cycle. According to the current study, compensated, but not decompensated congestive heart failure (CHF) is characterized by enhanced expression of myocardial ACE2 and downregulation of TMPRSS2. ADAM17 is responsible for shedding of ACE2, a process stimulated by AT1 receptor (AT1‐R) and may explain why renin angiotensin aldosterone system (RAAS) inhibitors augment ACE2 expression.
