Abstract
Since the discovery of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), numerous research has been undertaken to delineate the various effects of the virus which manifests in many ways all over the body. The association between the SARS‐CoV‐2 invasion mechanism and the renin‐angiotensin‐aldosterone system (RAAS) receptors, created many debates about the possible consequences of using RAAS‐modulating drugs including angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARBs) during the pandemic. Many clinical studies were conducted to assess the outcomes of coronavirus disease 2019 (COVID‐19) in patients who use ACEi/ARBs following the arguments claiming to discontinue these drugs as a precautionary measure. Although several studies mainly analyzed the outcomes of the disease, this review aimed to compare specific blood markers in both groups of COVID‐19 patients to gain better insight into the interaction of ACEi/ARBs with different body functions during the infection. Several databases were searched using a combination of keywords followed by screening and data extraction. Only 28 studies met our inclusion criteria, the majority of which showed no significant difference between the inflammation markers of COVID‐19 patients who used or did not use ACEi/ARBs. Interestingly, 6 studies reported lower inflammatory markers in COVID‐19 patients who used ACEi/ARBs, and 6 studies reported better outcomes among the same group. We therefore concluded that the use of ACEi/ARBs may not lead to worse prognosis of COVID‐19 and may even play a protective role against the hyperinflammatory response associated with COVID‐19.
Keywords: Angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, coronavirus, COVID‐19, renin angiotensin aldosterone system
The first cases of a novel coronavirus causing a pneumonia‐like infection surfaced in December 2019 in Wuhan, China. 1 Now almost a year later, the world is still suffering from the effects of this virus. The virus, later named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is the implicated pathogen in coronavirus disease 2019 (COVID‐19). To our present knowledge, there are 3 known coronaviruses capable of replicating in the lower respiratory tract causing pulmonary disease that can manifest in many ways and may be fatal in many cases. 1 They are called Middle East respiratory syndrome coronavirus (MERS‐CoV), severe acute respiratory syndrome coronavirus (SARS‐CoV), and SARS‐CoV‐2, which is responsible for the current pandemic
Early research showed a mechanism similar to that of SARS‐CoV but with more aggressive inflammatory responses damaging the airways as well as other organ systems. The sister viruses share about 79% of the same genome sequence. 2 The virus SARS‐CoV‐2, said to have zoonotic origins, has since dispersed quickly across the world and by March 24, 2020, had infected more than 381,000 people and taken the lives of 16,000. By January 23, 2021, COVID‐19 cases had gone up to more than 98 million worldwide, with more than 2.1 million deaths. 3 The scale of this pandemic calls for relevant research urgently, as professionals continue to develop efficient vaccines and communities attempt to maintain the proper protocols.
As alluded to before, SARS‐CoV‐2 seems to behave in a manner very similar to its close sister, SARS‐CoV. Coronaviruses in general require entry into host cells to cause disease. They achieve this through binding to certain cell‐surface receptors that then mediate endocytosis of the virus and successful entry into the cell. 4 The extensively studied SARS‐CoV was found to have a receptor‐binding domain (RBD) that recognizes and binds to angiotensin‐converting enzyme 2 (ACE2), which is an essential component of the renin‐angiotensin‐aldosterone system (RAAS) and is present in abundance in lung epithelia. 5 Interestingly enough, crystal analysis of the RBD of SARS‐CoV‐2 and its comparison with that of SARS‐CoV found unique differences that allow SARS‐CoV‐2 to bind with significantly higher affinity. 6
The RAAS system comprises multiple peptides, enzymes, and receptors that control plasma sodium concentration, extracellular volume, and arterial blood pressure (BP). 7 The liver releases angiotensinogen, which is hydrolyzed to angiotensin I via renin (released by the kidneys), and this happens during low arterial BP, low sodium chloride, or sympathetic nervous system activity. ACE, which is present in lungs, epithelium, and kidney, converts angiotensin I (ATI) to angiotensin II (ATII). This molecule then constricts the smooth muscle in arteries and veins and causes adrenal gland cortex to release aldosterone, which balances sodium‐potassium concentration in the kidney. 8 ATII can also stimulate the release of prostaglandins and promote lipogenesis, which influence renal vasoconstriction and increase adipose tissue mass, respectively. It has also been linked to glucose intolerance, insulin resistance, and adipose inflammation. ATII is broken down to angiotensin (1‐7) by ACE2, which is important for renal homeostasis. 9 Deficiency in ACE2 can lead to glomerular injury and albuminuria. Controlling RAAS to work with more ACE2 expression can help to balance endocrine functions. ACE2 is also present in multiple different organs in the body, such as the heart, testes, kidney, gut, lungs, liver, and upper respiratory airways. 10
Generally, ACE2 has been found to provide protection in cases of acute lung injury and is said to have a critical role in myocardial infarction, pulmonary and systemic hypertension, heart failure, and diabetic cardiovascular complications.10 This protective effect is most likely from ACE2 playing an integral role in the degradation of ATI and ATII, which have long been identified as causing deleterious effects on many organ systems through mechanisms such as vasoconstriction. 11 Dysregulation of RAAS can lead to multiple diseases such as hypertension, congestive heart failure, obesity, hepatic complications, kidney diseases, diabetes, ocular and neural diseases, and miscarriage. 7 More interestingly, however, is the mechanism by which ACE2 interacts with the immune system. Several studies have found that through the inactivation of ATII, ACE2 limits macrophage expression as well as proinflammatory cytokines, which in effect modulate the immune response. 12
The association between the SARS‐CoV‐2 invasion mechanism and the RAAS receptors created a dilemma regarding the possible consequences of using RAAS‐modulating drugs including the ACE inhibitors (ACEi) and angiotensin II receptor blockers (ARBs) during the pandemic. 13 Both ACEi and ARBs are widely used to treat hypertension and cardiovascular diseases (CVD). Several studies have shown evidence of increased ACE2 expression following the administration of a RAAS blockers. 14 , 15 , 16 , 17 This led to great concern among health care professionals regarding the possible role of RAAS inhibitors in increasing the risk and/or the severity of COVID‐19. Consequently, it was debated whether to continue or discontinue the medications during the pandemic. 17 However, withdrawing these drugs may lead to adverse health risks because of the uncontrolled blood pressure and/or untreated cardiac dysfunction. 18 , 19 Therefore, since the start of the pandemic, many researchers have investigated the effect of RAAS inhibitors on the primary outcomes of COVID‐19 by comparing the disease severity and mortality of COVID‐19 patients who use or do not use ACEi/ARBs. Several systematic reviews with meta‐analysis analyzed the results obtained from various clinical studies and reported no association between RAAS inhibitors and the increased risk or severity of COVID‐19. 20 , 21 These studies mainly analyzed data based on the outcomes of the disease including mortality, length of hospitalization, admission to the intensive care unit (ICU), and the use of mechanical ventilation in COVID‐19 patients who used or did not use ACEi/ARBs. Furthermore, it was reported that using RAAS inhibitors may improve the clinical outcomes of COVID‐19. 20 , 21
To the best of our knowledge, this is the first systematic review that compares the level of blood markers in COVID‐19 patients who used or did not use ACEi/ARBs rather than relying solely on the clinical outcomes. These blood markers include inflammation markers such as interleukin‐6 (IL‐6), C‐reactive protein (CRP), procalcitonin (PCT), ferritin, D‐dimer, cardiac markers such as brain natriuretic peptide (BNP), N‐terminal‐pro hormone BNP (NT‐proBNP), creatine kinase MB (CK‐MB), troponin, liver markers such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, renal markers such as creatinine, and tissue damage markers like lactate dehydrogenase (LDH). This is to gain better insight into the interaction of the RAAS inhibitors with the different body functions during the infection.
Methods
The protocol has not been registered; however, the preferred reporting items for systematic reviews and metanalysis (PRISMA) statement was used to develop the protocol of this systematic review. 22
Eligibility Criteria
We conducted a comprehensive literature search of cohort studies that compared laboratory results for COVID‐19 patients on ACEi/ARBs medications with those not on these medications. No restrictions were made about country, age, or sex. Any duplicated articles were removed, and articles that did not have any primary data such as review articles, were excluded from the study. During the full‐text screening, only studies that included inflammation marker blood tests for COVID‐19 patients who take RAAS inhibitors were selected. Studies that reported the inflammation marker blood tests for a mixed population of COVID‐19 patients that take or do not take RAAS inhibitors without splitting them into 2 distinctive groups were excluded, as the results were not conclusive for the purpose of this study. Furthermore, studies that reported the number or proportion of patients who had inflammation markers below or above certain values without formal statistical tests to assess the significance of the difference between the 2 groups were excluded.
Information Sources and Search Strategy
PubMed, Embase, Scopus, Google Scholar, Web of Science, Science Direct, Cochrane Library, and medRxiv databases were searched using combinations of the following keywords: severe acute respiratory syndrome coronavirus, severe acute respiratory syndrome‐coronavirus‐2, 2019‐ncov, 2019ncov, covid‐19, covid19, covid2019 ncov2019, ncov‐2019, hcov19, sars‐cov‐2, coronavirus, coronaviruses, coronavirus, coronaviruses, covid, hcov, coronavirus (MeSH terms), “Renin‐Angiotensin System” (Mesh), Renin, RAS, ACE2, angiotensin‐converting enzyme. The search strategy was appropriately adapted for each database. The following search strategy was used for searching PubMed: (“severe acute respiratory syndrome coronavirus 2” [Supplementary Concept] OR severe‐acute‐respiratory‐syndrome‐coronavirus‐2[Title/Abstract] OR 2019‐ncov[Title/Abstract] OR 2019ncov [Title/Abstract] OR covid‐19[Title/Abstract] OR covid19[Title/Abstract] OR covid2019[Title/Abstract] OR ncov2019[Title/Abstract] OR ncov‐2019[Title/Abstract] OR hcov19[Title/Abstract] OR sars‐cov‐2[Title/Abstract] OR coronavirus[Title/Abstract] OR coronaviruses[Title/Abstract] OR corona‐virus [Title/Abstract] OR corona‐viruses[Title/Abstract] OR covid[Title/Abstract] OR hcov[Title/Abstract] or “coronavirus” [MeSH Terms]) AND (“Renin‐Angiotensin System”[Mesh] or Renin OR RAS or ACE2 or angiotensin‐converting enzyme).
Study Selection and Data Collection
The systematic review was conducted using Covidence. 23 During the initial phase of screening, English, peer‐reviewed, and published articles (except for articles from medRxiv) from January 1 to December 17, 2020, were included, and all phases of screening and data extraction were conducted by 2 independent reviewers.
Data Items
Of the selected studies we collected the epidemiological, clinical, and laboratory data including age, sex, comorbidities, treatments and interventions, laboratory results, and outcomes for each group. Continuous variables were expressed as mean ± standard deviation or range of results. Categorical variables were expressed as percentages.
Risk of Bias and Quality Assessment
The quality of the included studies was assessed using the Newcastle‐Ottawa Quality Assessment Scale (NOS). Quality assessment was conducted by 2 independent reviewers.
Results
Results of search and screening are summarized in Figure 1. The PRISMA flow diagram shows the details of our protocol. After removing the duplicates, 339 of 5931 studies were selected for full‐text screening. Only 28 studies were eligible to be included in this review. The excluded studies included 105 studies irrelevant to the data we were looking for, 115 had ineligible outcomes or settings, 22 did not have enough data (no results for inflammation markers or no statistical analysis), 39 had no primary data, 15 were not in English, 4 were not accessible, 7 duplicates, 2 ongoing studies, and 2 retracted studies. All included studies had an NOS score of 5 or above.
Figure 1.
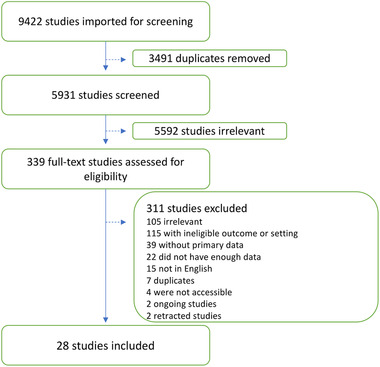
PRISMA flow diagram illustrating the study selection protocol.
This systematic review included a total of 28 studies all of which were retrospective cohort studies. All 28 studies separated the COVID‐19 patients into 2 groups: patients taking RAAS inhibitors and those taking other medications or not taking any. Supplementary Table S1 includes a summary of the demographic features of COVID‐19 patients for each study. 24 – 51 As reported in Supplementary Table S1, 8 studies had similar inclusion criteria for medication use, which was ACEi/ARBs use before admission that was continued after admission. Some studies had more specific criteria. For example, the chronic use of ACEi/ARBs was used as the inclusion criterion of the ACEi/ARBs group in the study conducted by Bae et al. 38 Adrish et al 36 included patients who used ACEi/ARBs during hospitalization. Lam et al 27 included patients who used ACEi/ARBs before admission. They conducted a separate analysis to assess the outcome of patients who continued or discontinued taking the medications during hospitalization. However, the blood marker results were not available for this type of analysis. Similarly, Chaudhri et al 48 compared patients who used ACEi/ARBs before hospitalization, regardless whether they continued or discontinued the medications during hospitalization with those who did not use ACEi/ARBs before hospitalization (part I). Then they compared the blood markers and outcomes of patients who continued ACEi/ARBs after admission with those who discontinued (part II). Some studies did not specify whether ACEi/ARBs were used before or after hospitalization or both.
The total number of COVID‐19 patients in all the studies was 7574, of whom 2723 were identified in each study as the ACEi/ARBs group. The ages of the patients were expressed as ranges or as mean values. The reported age ranges/means ranged from 32 to 86 and from 48 to 86.9 years in the ACEi/ARBs and control groups, respectively. Moreover, Supplementary Table S1 summarizes the reported underlying comorbidities and the P values reported by each study to determine any significant differences between the 2 groups of COVID‐19 patients who did or did not use ACEi/ARBs. Only 2 studies reported significantly higher proportions of the control patients with underlying chronic kidney disease (P = .001) 27 or dementia (P = .003). 41 However, both these studies as well as 15 other studies reported that the ACEi/ARBs groups had significantly higher proportions of patients with different comorbidities. The underlying comorbidities are summarized at the end of the results section in relation to any significantly different blood marker levels/outcomes between the 2 groups. No significantly different underlying comorbidities were reported in 11 studies. 32 , 33 , 34 , 35 , 37 , 42 , 43 , 44 , 45 , 46 , 47 , 51
Supplementary Table S2 summarizes the different treatments and interventions used for the 2 patient populations in the different studies. It also summarizes the blood test results for the various physiological markers being looked at in this review. This included inflammation markers such as IL‐6, CRP, PCT, ferritin, D‐dimer, cardiac markers such as BNP/NT‐proBNP, troponin, CK‐MB, liver markers such as ALT, AST, bilirubin, renal markers such as creatinine, and tissue damage markers such as LDH. Supplementary Table S3 shows the normal values for the different blood markers for reference. 52 , 53 , 54 The outcomes of each family of blood markers in COVID‐19 patients who did or did not use ACEi/ARBs in the included studies are detailed below.
Inflammation Markers (IL‐6, CRP, PCT, and Ferritin)
Of the 28 studies that reported the levels of blood markers, 22 studies showed no significant differences in the levels of CRP, PCT, or ferritin between the ACEi/ARBs group and the control groups. Only 6 studies reported significantly different levels of inflammatory markers between the 2 groups, all of which showed lower levels of inflammatory markers in the ACEi/ARBs group. Şenkal et al 32 reported lower CRP (P = .009) and ferritin (P = .025) in the ACEi group compared with the control group. Similarly, Yang et al 35 showed that CRP (P = .049) and PCT (P = .008) were lower in the ACEi/ARBs group compared with the control group. Chen et al 39 reported lower CRP (P = .0071) and IL‐6 (P = .0258) in the ACEi/ARBs group compared with the control group. Similarly, Meng et al 43 reported lower CRP (P < .001) in the ACEi/ARBs group compared with the control group. Yang et al 47 reported lower CRP and PCT (P = .049 and P = .008, respectively) in the ACEi/ARBs groups compared with the control group. In part II of the study conducted by Chaudhri et al, 48 CRP was lower (P = .03) in the ACEi/ARBs group, compared with the control group. CRP was higher (P = .02) in the ACEi/ARBs group compared with the control group in part I. However, an inflammatory score was created for each patient in both parts of the study by dividing the peak value of each inflammation marker by the admission value to produce what is called a fold‐change (FC) score. Quartiles were calculated and scored for the peak and FC values of each inflammation marker, and the sum of these scores was calculated across the different inflammation markers. No significant differences were observed in the FC scores between the ACEi/ARBs and the control groups in either part of the study. 48
Coagulation Marker (D‐Dimer)
Only 3 of 18 studies reported that the D‐dimer values significantly differed between the 2 groups. The D‐dimer was found to be higher (P = .002) in the ACEi/ARBs group compared with the control by Selçuk et al. 31 Lam et al 27 and Chen et al 39 reported lower D‐dimer (P = .003 and P = .0003, respectively) in the ACEi/ARBs group than in the control group.
Cardiac Markers (Troponin, CK‐MB, BNP/NT‐ProBNP)
Fifteen of 28 included studies reported the results of at least 1 cardiac blood marker in the ACEi/ARBs and control groups. Thirteen of such studies showed no significant difference between the 2 groups. Huang et al 26 reported that both troponin and NT‐proBNP were lower (P = .03 and P = .04, respectively) in the ACEi/ARBs group compared with the control group. Similarly, lower troponin (P = .005) and BNP (P = .01) were detected by Lam et al 27 in the ACEi/ARBs group compared with the control group.
Liver Markers (Bilirubin, ALT, AST)
Nineteen studies reported the laboratory results of at least 1 blood liver marker in the ACEi/ARBs and control groups. None of these studies reported any significant difference in the levels of bilirubin, ALT, or AST between the ACEi/ARBs and the control groups.
Kidney Marker (Creatinine)
Creatinine levels were reported in 22 studies, of which 18 studies did not report any significant difference between the ACEi/ARBs and control groups. López‐Otero et al, 29 Lim et al, 49 and Soleimani et al 50 found that the ACEi/ARBs group had higher creatinine (P = .019, P = .01, and P = .037, respectively) than the control group. Conversely, Wang et al found that the control group had higher creatinine (P = .047) than the ACEi/ARBs group.
Cell Damage Marker (LDH)
In 16 of the 19 studies that reported the LDH blood test results, no significant difference was observed in the LDH levels between the ACEi/ARBs and the control groups. Rossi et al 44 found that the LDH was higher in the ACEi/ARBs group (P = .009). Meng et al 30 and Chaudhri et al 48 (part II) reported lower LDH in the ACEi/ARBs group (P < .001 and P = .04, respectively).
Disease Outcomes (Severity and Mortality)
The outcome of COVID‐19 was assessed in most of the studies based on the mortality or the disease severity expressed as percentages of patients in each category. Of the 26 studies that reported the outcome, 18 studies reported no significant difference in the outcome of the disease between the ACEi/ARBs and the control groups. Selçuk et al 31 and Lim et al 49 reported that the ACEi/ARBs group showed higher mortality (P = .001 and P < .05, respectively) than the control group. Conversely, it was observed by Tan et al, 33 , Chen et al,39 Genet et al, 41 Meng et al, 43 Chaudhri et al 48 (part II), and Feng et al 51 that the ACEi/ARBs group showed lower mortality (P < .01, P = .0149, P = .03, P < .001, P = .04, and P = .037, respectively) compared with the control group.
Data were also collected to compare the treatments that each group of COVID‐19 patients received. López‐Otero et al 29 found that higher proportions of the ACEi/ARBs group received antiplatelets and anticoagulants (P < .001). Similarly, Bae et al, 38 Genet et al, 41 and Selçuk et al 31 reported that higher proportions of the ACEi/ARBs group received anticoagulants (P = .014), antiplatelets (P = .03), and aspirin (P = .03), respectively. Bae et al 38 and Wang et al 46 had higher proportions of the ACEi/ARBs groups admitted to the ICU (P = .02 and P = .041, respectively). Moreover, Selçuk et al 31 found that higher proportions of the ACEi/ARBs group required endotracheal intubation and admission to the ICU compared with the control group (P = .001 and P < .001, respectively). Conversely, Chaudhri et al 48 reported that a lower proportion of the ACEi/ARBs group was admitted to the ICU (P = .02). Şenkal et al 32 observed that a higher percentage of the ACEi/ARBs group received antibiotic (P < .001) and antiviral (P = .026) treatment. Furthermore, Yang et al 35 , 47 observed a contradicting result, with a lower proportion of the ACEi/ARBs group receiving antibiotic treatment. Similarly, Tan et al 33 reported lower proportions of the ACEi/ARBs group receiving corticosteroids or requiring noninvasive ventilation compared with the non‐ACEi/ARBs group (P = .003 and P = .048, respectively).
In many studies, multivariate regression analysis was conducted to adjust for confounding only to assess certain clinical outcomes. These outcomes included mortality, length of hospitalization, admission to the ICU, or need for mechanical ventilation. These data were reported in the additional information part of Supplementary Table S2 if available. However, most of the studies did not match the blood marker data to eliminate confounding. Therefore, comorbidities were taken into account to discuss the effect of using RAAS inhibitors on the levels of the different blood markers using the unmatched data. For all studies, the unmatched data are reported in Supplementary Table S2 for consistency, whereas the matched data are summarized whenever available in the conclusion and additional information part of the table. Furthermore, the conclusions made by the authors of each study have been summarized in Supplementary Table S2.
Summary of the Blood Marker/Outcome Results and the Underlying Comorbidities
The blood marker and outcome results are summarized in Figure 2. In general, the use of RAAS inhibitors did not increase the levels of inflammatory, cardiac, or liver markers in any of the studies. CRP was higher in the ACEi/ARBs group in part I of the study conducted by Chaudhri et al 48 ; however, it is not shown in Figure 2, as their calculated total inflammatory scores showed no significant difference between the 2 groups. In addition, significantly higher proportions of the ACEi/ARBs group in this part of the study had hypertension (HTN), coronary artery disease (CAD), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD). 48 Lower inflammation markers in the ACEi/ARBs group were reported by 6 studies. 32 , 35 , 39 , 42 , 43 , 48 Furthermore, the ACEi/ARBs group had a higher proportions of patients with comorbid coronary heart disease (CHD) 39 in 1 study. Higher levels of coagulation, 31 kidney, 29 , 49 , 50 and cell damage 44 markers were reported in the ACEi/ARBs group as compared with the control group in 5 studies, all of which reported that the ACEi/ARBs group had higher proportions of patients with comorbidities (CAD, 31 CVD/dyslipidemia/diabetes/obstructive sleep apnea‐hypopnea syndrome/HTN/ obesity/stroke/CKD,29 HTN, 49 CVD, 50 COPD 44 ). Lower levels of coagulation, 27 , 39 cardiac, 26 , 27 kidney, 46 and cell damage 43 , 48 markers were reported in the ACEi/ARBs group in 6 studies, 2 of which reported higher proportions of ACEi/ARBs patients with comorbid CHD 39 and diabetes. 27 Only 2 of the 6 studies reported that the control group had higher proportions of patients with CKD 27 and older age. 26 The ACEi/ARBs group had a significantly higher mortality rate in 2 studies. 31 , 49 However, both studies reported that the ACEi/ARBs group had higher proportions of patients with HTN 49 and CAD. 31 Lower mortality was observed in the ACEi/ARBs group in 6 studies, 33 , 39 , 41 , 43 , 48 , 51 which also had significantly higher proportions of ACEi/ARBs patients with underlying CAD/HTN41 and CHD 39 in 2 studies.
Figure 2.
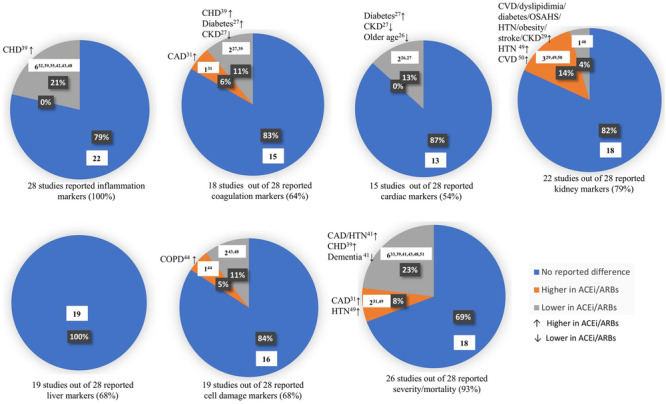
Summary of the blood marker and outcome results in the 28 included studies. Each pie chart illustrates the percentage and number of studies that reported no significant difference, significantly higher, or significantly lower blood marker/outcome in the ACEi/ARBs group compared with the control group. The significantly different proportions in patients with underlying comorbidities between the 2 groups in the studies that reported significantly different levels of markers/outcomes are reported next to each sector. The use of RAAS inhibitors did not increase the levels of inflammatory, cardiac, or liver markers in any of the studies. Lower inflammation markers in the ACEi/ARBs group were reported by 6 studies, 32 , 35 , 39 , 42 , 43 , 48 1 of which reported higher proportions of the ACEi/ARBs group with comorbid CHD. 39 Higher levels of coagulation, 31 kidney, 29 , 49 , 59 and cell damage 44 markers were reported in the ACEi/ARBs group in 5 studies. However, in all these studies, the ACEi/ARBs group had higher proportions of patients with comorbidities. Lower levels of coagulation, 27 , 39 cardiac, 26 , 27 kidney, 46 and cell damage 43 , 48 markers were reported in the ACEi/ARBs group in 6 studies. Higher proportions of the ACEi/ARBs group had comorbid CHD 39 and diabetes 27 in 2 of the 6 studies. Only 2 studies reported that the control group had higher proportions of patients with chronic kidney disease (CKD) 27 and older age. 26 The ACEi/ARBs group had significantly higher mortality rate in 2 studies, 31 , 49 which could be attributed to the higher proportions of patients with hypertension (HTN) 49 and coronary artery disease (CAD). 31 Interestingly, 6 studies 33 , 39 , 41 , 43 , 48 , 51 reported lower mortality in the ACEi/ARBs group, who had significantly higher proportions of patients with underlying HTN/CAD41 and coronary heart disease (CHD) 39 in 2 studies.
Discussion
This systematic review included 28 articles that reported the demographic and clinical data of 7628 COVID‐19 patients who either did or did not use RAAS inhibitors. There is accumulating evidence suggesting that the mortality and severe outcomes of COVID‐19 are associated with elevated inflammatory mediators. This hyperinflammation results from dysregulated host innate immune response, leading to what is called the cytokine storm. 55 , 56 , 57 For this reason, it was essential to compare the level of some blood inflammation markers in the ACEi/ARBs and the control groups as an important indicator of prognosis.
Inflammation and Coagulation Markers
Previous studies have shown inflammatory markers such as CRP, IL‐6, and ferritin to be significantly elevated in COVID‐19 patients. 58 Elevated CRP levels were strongly linked to poor prognosis of COVID‐19 and were reported to be a tool to predict the outcome of the diseases. 59 One study found that ferritin levels are related to the severity of COVID‐19, which is directly related to the hyperinflammation response as a result of the infection. 60 PCT, which is commonly used to differentiate bacterial from viral infections, was also found to be associated with severe cases of COVID‐19 and could be used as a useful tool for predicting the prognosis. 61 In this review, only Chaudhri et al 48 (part I) reported significantly higher levels of CRP in the ACEi/ARBs group than the control group. This could be attributed to the observed significantly higher proportions of patients in the ACEi/ARBs group who had comorbid diabetes, HTN, heart failure (HF), CKD, and COPD. This was confirmed by multivariate analysis, which revealed that using ACEi/ARBs during hospitalization was associated with a reduction in the inflammatory burden. Furthermore, when multiple inflammation markers were considered, comparable peak inflammation, and FC scores were obtained for both groups. 48 Interestingly, Şenkal et al, 32 Yang et al, 35 , 47 Chen et al, 39 Meng et al, 43 and Chaudhri et al 48 (part II) found that patients who used RAAS inhibitors had significantly lower CRP, ferritin, and/or PCT. No significant difference in the comorbidities was reported between the 2 groups except for Chen et al, 39 who observed more patients with CHD in the ACEi/ARBs group. These 3 markers are known as acute‐phase reactants, and their levels increase during periods of inflammation under the influence of IL‐6 and other inflammatory cytokines. This could be linked to the immunomodulatory role of RAAS inhibitors by upregulating the expression of ACE2.
The role of the RAAS in the immune system has long been studied and established. ACEi were reported to reduce the Th1/Th2 cytokines ratio and consequently modulate inflammation. 62 ATII has been found to mediate inflammatory responses through upregulation of many proinflammatory receptors such as E‐selectin and P‐selectin. 63 Furthermore, ATII has been implicated in the increase in production of reactive oxygen species (ROS) through the activation of NADPH oxidase. 63 ATII has also been found to trigger the expression of toll like receptors by many cells as well as induce dendritic cell maturation. 63 All these proinflammatory effects of ATII lead to the hypothesis that the upregulation of ACE2 can modulate the immune response through its main action of degrading ATII. 64 This may play a significant role during infection with SARS‐CoV‐2, which is known to cause a hyperactive immune response that can get out of check and proceed to harm many organs in the body, causing the many systemic effects observed in the COVID‐19 era. It was found that mice deficient in the ACE2 gene had significantly more severe acute respiratory distress syndrome (ARDS); when infected with SARS‐CoV‐2 compared with the wild type. Loss of ACE2 expression with SARS‐CoV‐2 infection caused higher vascular permeability and lung edema. Interestingly, treatment with catalytically active recombinant ACE2 protein led to symptoms improving. 65 , 66 Several studies from the SARS‐CoV era as well as some during this pandemic have shown that these viruses can cause downregulation of the ACE2 receptor. 67 This downregulation of ACE2 disturbs the balance in the RAAS, favoring increased ATII and therefore a heightened inflammatory response. 12 It would be interesting to be able to quantify the exact levels of the different RAAS components during a COVID‐19 infection to further test this hypothesis. This distortion in the balance of the RAAS toward the proinflammatory side could very well be what causes such severe disease in patients. Thus, it would seem that anything that can upregulate ACE2 and block ATII might have the potential to tip the balance back to normal and modulate the immune response. Interestingly enough, RAAS inhibitors have been shown to cause upregulation of ACE2 in many studies. 14 This could provide an explanation for why Şenkal et al, 32 Chen et al, 39 Meng et al, 43 Chaudhri et al, 48 and Yang et al 35 , 47 observed significantly decreased inflammatory markers in patients who used RAAS inhibitors. This contradicts the preliminary assumption that RAAS inhibitors would predispose patients to a higher likelihood of infection or worse outcomes because of the upregulation of ACE2, which facilitates the invasion of SARS‐CoV‐2.
The D‐dimer is produced during the cleavage of fibrin by plasmin to break down clots. D‐dimer has been identified as a biomarker for disease severity and mortality in COVID‐19 patients. It is still unclear whether this association is attributed to the direct effect of SARS‐CoV‐2 or to the consequences of systemic inflammatory response. 68 Interestingly, Human and mouse platelets were found to express ACE2. Zhang et al 69 reported that SARS‐CoV‐2 binds platelet ACE2 and enhances thrombosis in COVID‐19 patients. SARS‐CoV‐2 was found to promote ACE2 internalization and degradation, which was also observed as a gradual reduction in the expression of ACE2 in platelets in COVID‐19. Furthermore, SARS‐CoV‐2 directly activates the ACE2/mitogen‐activated protein kinase pathway to potentiate platelet activation and aggregation. This was found to be reversed by using recombinant human ACE2 and an antispike monoclonal antibody that suppress SARS‐CoV‐2‐induced platelet activation. 69 Only 3 studies reported significantly different D‐dimer levels in the ACEi/ARBs and the control groups. Selçuk et al 31 found that values for D‐dimer were significantly higher in patients who took RAAS inhibitors. This could be attributed to the significantly higher number of patients with comorbid conditions in the RAAS inhibitor group compared with the control group, which may predispose that group to worse outcomes. Lam et al 27 reported significantly lower D‐dimer in the ACEi/ARBs group compared with the control group. However, The ACEi/ARBs group had a higher proportion of comorbid diabetes, whereas the control group had a significantly higher proportion of patients with CKD. Chen et al 39 reported significantly lower D‐dimer in the ACEi/ARBs group, who also had a significantly higher proportion of patients with comorbid CHD compared with the control group. Furthermore, RAAS inhibitors did not cause any significant increase in D‐dimer in the patients in the ACEi/ARBs group compared with the control group in 15 studies. Further investigations are required to understand how RAAS inhibitors may affect the level of D‐dimer and whether this effect is attributed to the upregulation of ACE2 in platelets and/or to modulating inflammation.
Cardiac Markers
It was reported that COVID‐19 may cause cardiac injury even to those who do not have any underlying CVD. An early study coming out of Wuhan, China, found that some of the causes of death in COVID‐19 patients included fatal arrhythmias, acute coronary syndrome, and cardiac arrest. 70 Both troponins and BNP have been found to be elevated in patients with COVID‐19. 71 It has since been extensively argued whether the reported cardiac injury is because of COVID‐19 or becaue of the underlying comorbidities that affected many of the COVID‐19 patients in the reported studies. 72 A study looking at biopsy specimens from COVID‐19 patients with myocarditis suggests that the degree of cardiac inflammation and macrophage infiltration was too high to be accounted for simply by viral invasion and theorizes that the pathological process taking place in the heart is a mixture of the ramped‐up immune response associated with COVID‐19 and underlying heart diseases. 73 In our review, 2 studies (Huang et al 26 and Lam et al 27 ) revealed that the ACEi/ARBs group had a significantly lower level of cardiac markers (troponin, BNP, or NT‐pro‐BNP). In the study conducted by Huang et al, the ACEi/ARBs and the control groups had different proportions of patients with different comorbidities, but the differences did not reach the level of statistical significance. However, they reported that a significantly higher proportion of patients in the control group were older than 65 years. Therefore, Huang et al 26 concluded that the level of cardiac markers was not significantly different in the 2 groups after excluding the age factor. Lam et al 27 reported comparable proportions of patients in both groups with CHD/HF. However, the ACEi/ARBs group had a higher proportion of patients with comorbid diabetes, which does not justify the significantly lower levels of cardiac markers. Conversely, it was reported that elevated serum concentrations of cardiac biomarkers were detected in patients with type 2 diabetes because of the higher risk of developing CVD. 74 , 75 , 76 Furthermore, after correction for multiple comparisons, Lam et al 27 found that troponin level remained significantly different between the ACEi/ARBs and the control groups.
It is also important to note that patients taking RAAS inhibitors are more likely to have underlying heart disease that would predispose this population to worse cardiac outcomes with a COVID‐19 infection. However, none of the included studies reported that the ACEi/ARBs group had significantly higher levels of cardiac markers than the control group. This can be attributed to the immunomodulatory effect of RAAS inhibitors during infection. 47 As it is now established that inflammation plays an important role in the progression and outcome of COVID‐19, it may also lead to different types of cardiac dysfunctions including myocardial infarction and heart failure. 77 Troponins are enzymes released from cardiac myocytes when they are injured. It could then be hypothesized that because RAAS inhibitors have been shown to modulate the immune response, their use can decrease systemic inflammation, which would protect cardiac myocytes from inflammatory injury. BNP is a peptide released from cardiac myocytes when they experience excessive stretch such as during states of volume overload and is generally used as a surrogate for cardiac function. The more the heart loses its contractile function, the more stretched it becomes and thus releases more BNP, which functions as part of the body's main mechanism to counteract RAAS by promoting natriuresis and preventing excessive volume overload. RAAS inhibitors have long been established to have a cardioprotective effect in patients with CVD. 78 This mechanism may explain the lower levels of cardiac markers in the ACEi/ARBs group reported by Lam et al. 27 Furthermore, it suggests that RAAS inhibitors may play a cardioprotective role against the COVID‐19‐associated cardiac injury that has been widely reported. 79
Other Blood Markers
In addition to cardiac injury, it was reported that COVID‐19 may lead to multiple organ failure, which could be mainly attributed to the severe inflammatory response associated with the virus that attacks many organs in the body and leads to dysfunction. 80 In addition to the inflammatory and cardiac markers, our review attempted to look at other markers of organ function such as the kidney and liver function in COVID‐19 patients. Wang et al 46 reported significantly lower levels of creatinine in the ACEi/ARBs group compared with the control group, and no difference between the 2 groups in comorbidities was reported. López‐Otero et al, 29 Lim et al, 49 and Soleimani et al 50 found that creatinine was significantly elevated in patients who took RAAS inhibitors compared to those in the control group. However, the comorbidity data in the RAAS inhibitor group had a significantly higher number of patients with kidney disease, CVD, diabetes, obesity, and/or stroke. In general, RAAS inhibitors were found to be associated with increased serum creatinine. 81 RAAS inhibitors impair the effect of ATII by either preventing its formation (ACEi) or blocking its receptor (ARBs). Angiotensin II is known to constrict the afferent and efferent arterioles, with a preference for increasing the resistance of the efferent arteriole. Therefore, RAAS inhibitors decrease the resistance at the postglomerular arteriole, which lowers the intraglomerular pressure and reduces the glomerular filtration rate. This can be caused by volume depletion because of overaggressive diuresis in CKD and HF patients. 82 However, as RAAS inhibitors are antihypertensive drugs, they can slow the rate of progression of nephropathy in both diabetic and nondiabetic CKD patients. RAAS inhibitors may reduce the intraglomerular pressure and hyperfiltration and reduce proteinuria, which impairs the progression of CKD. 83
Several studies revealed that RAAS inhibitors offer beneficial therapeutic strategies to treat and prevent chronic liver disease and portal hypertension. ATII was found to induce various profibrotic pathways by binding to its receptors expressed in different types of organs such as heart, kidney, and liver. Activation of these receptors may enhance the transformation of the quiescent hepatic stellate cells (HSCs) into myofibroblast‐like activated HSCs and the production of profibrotic cytokines. RAAS inhibitors were reported to reduce the proliferation of HSCs to reduce the profibrotic molecules that may improve liver fibrosis. 84 None of the included studies reported that using RAAS inhibitors significantly increased any of the liver markers in the ACEi/ARBs group compared with the control group.
ATII can induce apoptosis and the release of inflammatory mediators leading to cell damage. Zhang et al 85 demonstrated that ATII‐induced damage of human umbilical vein endothelial cells. This damaging effect because of apoptosis was counteracted by the addition of human recombinant ACE2. Furthermore, the production of the cell damage marker LDH was significantly lower in the presence of ACE2. Therefore, RAAS inhibitors may play a similar protective role by upregulating the ACE2 levels and blocking the apoptotic and damaging effect of ATII. This may explain the results obtained by Meng et al43 and Chaudhri et al 48 (part II), who reported significantly lower LDH in the ACEi/ARBs group than in the control group. Both groups had statistically comparable proportions of patients with comorbidities. Conversely, Rossi et al 44 found significantly higher LDH in the ACEi/ARBs group. This can be attributed to the underlying COPD, which was reported in a significantly higher proportion of the ACEi/ARBs group. The LDH level was reported to be elevated in patients with COPD because of the oxidative metabolic response of the muscles to compensate for the ventilation abnormalities. 86 No other significant differences were observed in LDH levels between the ACEi/ARBs and control groups in the included studies.
Disease Outcomes (Severity and Mortality)
Although ACEi and ARBs affect RAAS through different mechanisms, it was reported that their impact on the outcomes of COVID‐19 does not significantly differ. A large observational study conducted by the Cleveland Clinic 87 that compared patients on ACEi with those on ARBs found no significant difference in the rates of hospitalization, ICU admission, or need for a ventilator. This aligns with what was mentioned before about ATII being the main effector driving an out‐of‐control immune response. Both ACEi and ARBs reduce the effects of ATII by either inhibiting the conversion of ATI to ATII or by blocking the ATII receptors, respectively. 88 Multivariable analysis conducted by Selçuk et al 31 revealed that the use of RAAS inhibitors was an independent predictor of inhospital mortality, as a significantly higher proportion of the ACEi/ARBs group died in the hospital. They also reported a higher rate of ICU admission within the same group. No significant difference was observed in the blood inflammatory and physiology markers except for the D‐dimer, which was significantly higher in the ACEi/ARBs group. Accordingly, Selçuk et al 31 concluded that ACEi/ARBs therapy might not play any beneficial role for hypertensive COVID‐19 patients. However, it is worth noting that the ACEi/ARBs group had a significantly higher ratio of comorbid CAD. Similarly, Lim et al 49 reported significantly higher mortality in the ACEi/ARBs group, which had a significantly higher proportion of patients with underlying HTN. Their multivariate logistic regression analysis for severe complications, which adjusted some confounders (not including HTN), revealed that ACEi/ARBs had a significant association with ARDS and acute kidney injury. However, they concluded that ACEi/ARBs were associated with increased risk of inhospital mortality and severe complications such as ARDS. Significantly higher proportions of the ACEi/ARBs groups were admitted to the ICU in the studies conducted by Bae et al 38 and Wang et al. 46 However, their propensity score matching to eliminate confounders revealed no significant different between the 2 groups of patients. Conversely, Chaudhri et al 48 (part II) reported that a significantly lower proportion of the ACEi/ARBs group required admission to the ICU.
Of the 28 included studies, 26 reported no other evidence indicating a worse outcome of COVID‐19 patients who used any of the 2 types of medication. Furthermore, in addition to their reported significantly lower cardiac markers associated with the chronic use of ACEi/ARBs compared with the control group, Lam et al 27 compared the outcomes of patients who continued taking the drugs in the hospital with those who discontinued. Interestingly, patients who continued ACEi/ARBs after admission had a significantly lower rate of ICU admission and mortality. Moreover, Tan et al, 33 Chen et al,39 Genet et al, 41 Meng et al,43 and Chaudhri et al 48 reported a significantly lower death rate in the ACEi/ARBs group compared with the control group. This could be attributed to the previously described protective role that RAAS inhibitors play in many body organs. Of the 26 included studies, all but those of Selçuk et al 31 and Lim et al 49 stated in their conclusions that RAAS inhibitors improved the outcomes (10 studies) or were not associated with worse outcomes of COVID‐19 (16 studies). This is further supported by the findings of the BRACE CORONA trial, which is the first randomized trial comparing the effect of continuing versus stopping ACEi/ARBs in COVID‐19 patients. The trial enrolled more than 659 patients from 34 sites in Brazil, and the eligible patients were randomized to temporarily suspend or continue to use ACEi. Median days alive and out of the hospital at 30 days were the primary outcomes, whereas the secondary outcomes included progression of COVID‐19 disease, all‐cause mortality, death from cardiovascular causes or cardiovascular complications such as troponin, BNP, N‐proBNP, and D‐dimer levels, myocardial infraction, stroke and thromboembolic events. 89 , 90 , 91 The principal findings of the BRACE CORONA trial were presented at the European Society of Cardiology Virtual Congress, September 1, 2020. The findings revealed no significant difference in days alive out of the hospital and all‐cause death at 30 days between the ACEi/ARBs group and patients who stopped using ACEi/ARBs. Furthermore, no significant difference was observed between the 2 groups in common adverse events such as respiratory failure requiring IMV, shock requiring vasopressors, acute myocardial infarction or worsening heart failure and acute kidney failure requiring hemodialysis. Therefore, suspending ACEi/ARBs was not beneficial, and it did not improve days alive and out of the hospital. 89 , 90 , 91
In summary, the use of RAAS inhibitors did not increase inflammatory, cardiac, or liver markers in any of the studies. CRP was higher in the ACEi/ARBsgroup in part I of the study conducted by Chaudhri et al 48 ; however, their calculated total inflammatory scores showed no significant difference between the 2 groups. In addition, significantly higher proportions of the ACEi/ARBs group in this part of the study had HTN, CAD, CKD, and COPD. 48 Lower inflammation markers in the ACEi/ARBs group were reported in 6 studies. 32 , 35 , 39 , 42 , 43 , 48 Five of these studies did not report any significantly different comorbidities between the 2 groups. Furthermore, the ACEi/ARBs group had a higher proportion of patients with comorbid CHD 39 in 1 study. These results suggest that RAAS inhibitors may play an immunomodulatory effect, concurring with the findings of other studies. 47 Higher levels of coagulation, 31 kidney, 29 , 49 , 59 and cell damage 44 markers were reported in the ACEi/ARBs group compared with the control group in 5 studies. However, in all these studies, the ACEi/ARBs group had higher proportions of patients with several comorbidities. 29 , 31 , 44 , 49 , 50 Lower levels of coagulation, 27 , 39 cardiac, 26 , 27 kidney, 46 and cell damage 43 , 48 markers were reported in the ACEi/ARBs group in 6 different studies, 2 of which reported higher proportions of ACEi/ARBs patients with comorbid CHD 39 and diabetes. 27 Only 2 of the 6 studies reported that the control group had higher proportions of patients with CKD 27 and older age. 26 The ACEi/ARBs group had a significantly higher mortality rate in 2 studies, 31 , 49 which could be attributed to the higher proportions of patients with HTN 49 and CAD. 31 Interestingly, lower mortality was observed in the ACEi/ARBs group in 6 studies, 33 , 39 , 41 , 43 , 48 , 51 which also had significantly higher proportions of ACEi/ARBs patients with underlying CAD/HTN41 and CHD 39 in 2 studies, possibly indicating an improved outcome. With the exception of inflammation markers, not all the included studies reported results on blood markers. Of the studies that reported inflammatory, coagulation, cardiac, kidney, liver, and cell damage markers, 79%, 83%, 87%, 82%, 100%, and 84% did not show any significant difference between the ACEi/ARBs and the control groups. Moreover, 69% of the studies that reported disease outcome (severity/mortality) observed no significant difference between the 2 groups.
It was challenging to link the timing of using the medications with the reported results. Although some articles stated clearly that ACEi/ARBs were used before hospitalization but nothing was mentioned about the inhospital use. It is possible that among those who were classified as ACEi/ARBs groups based on their chronic use of the medications, some may have continued and some discontinued using the medications during hospitalization. Similarly, some studies stated clearly that ACEi/ARBs were used during hospitalization without specifying the history of usage. It is also possible that some patients were using the medications before admission. This may create an overlap between all 3 groups of studies that classified their ACEi/ARBs groups based on using the medications before, during, or before and during hospitalization. Furthermore, 24% of the studies did not clearly explain the timing of ACEi/ARBs usage.
Limitations
There are some limitations in this review. Some studies clearly defined the ACEi/ARBs group. However, it was not clear in many others whether the ACEi/ARBs groups used the RAAS inhibitors before hospitalization, during hospitalization, or both. Another limitation was the large difference in the number of patients in the ACEi/ARBs and the control groups, with the former usually having a smaller number of COVID‐19 patients. Furthermore, some studies compared the clinical outcomes in hypertensive patients taking ACEi/ARBs with those taking other types of drugs. However, in many studies, patients in the control group did not always have HTN. Multivariate regression analysis was conducted in some studies to adjust for cofounding only to assess certain clinical outcomes. These outcomes included mortality, length of hospitalization, admission to the ICU, and need for mechanical ventilation. However, most of the studies did not match the blood marker results to eliminate confounding.
Conclusion
There is accumulating evidence that RAAS inhibitors do not have any harmful effect on COVID‐19 patients. This is also evident from the data collected in this review, which indicate no significantly higher inflammation markers found to be linked to a worse disease prognosis. Moreover, using RAAS inhibitors was not found to be linked to any significantly higher physiological blood markers or bad prognosis of COVID‐19 or even mortality in most of the studies. This is in agreement with several other studies including the BRACE CORONA trial, 89 , 90 , 91 , 92 , 93 , 94 which reported that the suspension of ACEi/ARBs was not associated with better outcomes and/or using the medications was not associated with worse outcomes.
In summary, the use of RAAS inhibitors may not lead to higher disease markers or worse prognosis or mortality. Furthermore, several results suggested an added benefit and a protective effect of RAAS inhibitors. Therefore, it is our recommendation to not discontinue RAAS inhibitors out of unfounded fear of increased susceptibility to infection. This is the same recommendation adopted by the major professional societies such as the American College of Cardiology. Suspension of these drugs can lead to significant harm to patients with comorbid conditions in which an ACEi/ARBs is a primary indication and, in some diseases, can improve mortality. We also see importance in further testing of RAAS inhibitors in patients without comorbid conditions who are not already using these medications to utilize their possible added protective effect through immune modulation during COVID‐19 infection, which warrants further trials and investigation.
Conflicts of Interest
Mohamed B. Elshazly is a cofounder of EMBER Medical Telemedicine company, and he owns equity in this company, which is irrelevant to this research. The other authors declare no conflicts of interest.
Funding
No fund was received for this study.
Data‐Sharing Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Acknowledgments
We thank Mr. Sa'ad Laws for his help during the early and late phases of this project including developing the search strategy and importing papers. We also thank Weill Cornell Medicine‐Qatar for its continuous support.
Hiba Naveed and Abdallah Elshafeey contributed first co‐authors.
Emmad Janjua, Areej Nauman, Hussam Kawas, and Ridhima Kaul contributed equally to this study.
References
- 1. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns Hopkins University . Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center. Baltimore, MD: Johns Hopkins University; 2020. [Google Scholar]
- 4. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin‐ and caveolae‐independent endocytic pathway. Cell Res. 2008;18(2):290‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS‐CoV‐2. Proc Natl Acad Sci U S A. 2020;117(21):11727‐11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel S, Rauf A, Khan H, Abu‐Izneid T. Renin‐angiotensin‐aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317‐325. [DOI] [PubMed] [Google Scholar]
- 8. Fountain JH, Lappin SL. Physiology, Renin Angiotensin System. https://www.ncbi.nlm.nih.gov/books/NBK470410/. Published 2019. Accessed November 2020. [Google Scholar]
- 9. Ferrario CM. Angiotension‐(1‐7) and antihypertensive mechanisms. J Nephrol. 1998;11(6):278‐283. [PubMed] [Google Scholar]
- 10. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126(10):1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel VB, Zhong J‐C, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 Axis of the renin–angiotensin system in heart failure. Circ Res. 2016;118(8):1313‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crowley SD, Rudemiller NP. Immunologic effects of the renin‐angiotensin system. J Am Soc Nephrol . 2017;28(5):1350‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296(2):F398‐F405. [DOI] [PubMed] [Google Scholar]
- 15. Milne S, Yang CX, Timens W, Bossé Y, Sin DD. SARS‐CoV‐2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med. 2020;8(6):e50‐e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 17. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan MS, Fonarow GC, Ahmed A, et al. Dose of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers and outcomes in heart failure: a meta‐analysis. Circ Heart Fail. 2017;10(8):e003956. [DOI] [PubMed] [Google Scholar]
- 19. Blood pressure lowering treatment trialists’ collaboration. Blood pressure dependent and independent effects of agents that inhibit the renin–angiotensin system. J Hypertens. 2007;25(5):951‐958. [DOI] [PubMed] [Google Scholar]
- 20. Pranata R, Permana H, Huang I, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14(5):983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22(10):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 23. Covidence Systematic Review Software, Melbourne, Australia: Veritas Health Innovation. www.covidence.org. Accessed January 2021. [Google Scholar]
- 24. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. [DOI] [PubMed] [Google Scholar]
- 25. Hu J, Zhang X, Zhang X, et al. COVID‐19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome. J Infect. 2020;81(6):979‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8(7):430‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lam KW, Chow KW, Vo J, et al. Continued in‐hospital angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID‐19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for Coronavirus Disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. López‐Otero D, López‐Pais J, Cacho‐Antonio CE, et al. Impact of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on COVID‐19 in a western population. CARDIOVID registry. Rev Esp Cardiol (Engl Ed). 2020;74(2):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Selçuk M, Çınar T, Keskin M, et al. Is the use of ACE inb/ARBs associated with higher inhospital mortality in Covid19 pneumonia patients? Null. 2020;42(8):738‐742. [DOI] [PubMed] [Google Scholar]
- 32. Şenkal N, Meral R, Medetalibeyoğlu A, Konyaoğlu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan N‐D, Qiu Y, Xing X‐B, Ghosh S, Chen M‐H, Mao R. Associations between angiotensin‐converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID‐19. Gastroenterology. 2020;159(3):1170‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu J, Huang C, Fan G, et al. Use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID‐19 outbreak: a retrospective analysis. Front Med. 2020;14(5):601‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang G, Tan Z, Zhou L, et al. Effects of angiotensin II receptor blockers and ace (angiotensin‐converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID‐19 and hypertension. Hypertension. 2020;76(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 36. Adrish M, Chilimuri S, Sun H, Mantri N, Yugay A, Zahid M. The association of renin‐angiotensin‐aldosterone system inhibitors with outcomes among a predominantly ethnic minority patient population hospitalized with COVID‐19: the bronx experience. Cureus. 2020;12(9):e10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anzola GP, Bartolaminelli C, Gregorini GA, et al. No harm from angiotensin‐converting enzyme inhibitors or angiotensin receptor inhibitors in patients with COVID‐19. Results of a prospective study on a hospital‐based cohort. Ital J Med. 2020;14(3):162‐166. [Google Scholar]
- 38. Bae DJ, Tehrani DM, Rabadia SV, et al. Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID‐19. Am J Cardiol. 2020;132:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen C, Wang F, Chen P, et al. Mortality and pre‐hospitalization use of renin‐angiotensin system inhibitors in hypertensive COVID‐19 patients. J Am Heart Assoc. 2020;9(21):e017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cui H, Wu F, Fan Z, Cheng X, Cheng J, Fan M. The effects of renin–angiotensin system inhibitors (RASI) in coronavirus disease (COVID‐19) with hypertension: a retrospective, single‐center trial. Med Clín. 2020;155(7):295‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Genet B, Vidal J‐S, Cohen A, et al. COVID‐19 In‐hospital mortality and use of renin‐angiotensin system blockers in geriatrics patients. J Am Med Dir Assoc. 2020;21(11):1539‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khan KS, Reed‐Embleton H, Lewis J, Bain P, Mahmud S. Angiotensin converting enzyme inhibitors do not increase the risk of poor outcomes in COVID‐19 disease. A multi‐centre observational study. Scott Med J. 2020;65(4):149‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meng X, Liu Y, Wei C, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers improved the outcome of patients with severe COVID‐19 and hypertension. Sci China Life Sci. 2020;2020:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rossi L, Malagoli A, Biagi A, et al. Renin–angiotensin system inhibitors and mortality in patients with COVID‐19. Infection. 2020;2020;22:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sardu C, Maggi P, Messina V, et al. Could Anti‐hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID‐19 infection? Data from centers of Southern Italy. J Am Heart Assoc. 2020;9(17):e016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Z, Zhang D, Wang S, et al. A retrospective study from 2 centers in China on the effects of continued use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in patients with hypertension and COVID‐19. Med Sci Monit. 2020;26:e926651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang G, Tan Z, Zhou L, et al. Angiotensin II Receptor Blockers and Angiotensin‐Converting Enzyme Inhibitors Usage is Associated with Improved Inflammatory Status and Clinical Outcomes in COVID‐19 Patients With Hypertension. https://www.medrxiv.org/content/10.1101/2020.03.31.20038935v1. Accessed December 2020. [DOI] [PubMed]
- 48. Chaudhri I, Koraishy FM, Bolotova O, et al. Outcomes associated with the use of renin‐angiotensin‐aldosterone system blockade in hospitalized patients with SARS‐CoV‐2 infection. Kidney360. 2020;1(8):801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lim J‐H, Cho J‐H, Jeon Y, et al. Adverse impact of renin–angiotensin system blockade on the clinical course in hospitalized patients with severe COVID‐19: a retrospective cohort study. Sci Rep. 2020;10(1):20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Soleimani A, Kazemian S, Karbalai Saleh S, et al. Effects of Angiotensin Receptor Blockers (ARBs) on in‐hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID‐19. Am J Hypertens. 2020;33(12):1102‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng Z, Li J, Yao S, et al. Clinical factors associated with progression and prolonged viral shedding in COVID‐19 patients: a Multicenter Study. Aging Dis. 2020;11(5):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Padilla O. Blood Tests: Normal Values ‐ Resources. MSD Manual Professional Edition; 2018. [Google Scholar]
- 53. IL6 ‐ Clinical: Interleukin 6, Plasma. IL6. http://www.mayocliniclabs.com. https://www.mayocliniclabs.com/test‐catalog/Clinical%B1;and%B1;Interpretive/63020. Accessed November 20, 2020. [Google Scholar]
- 54. PCT ‐ Clinical: Procalcitonin, Serum. PCT. www.mayocliniclabs.com. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/83169. Accessed November 20, 2020. [Google Scholar]
- 55. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID‐19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID‐19. Am J Pathol. 2020;191(1):4‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galván‐Román JM, Rodríguez‐García SC, Roy‐Vallejo E, et al. IL‐6 serum levels predict severity and response to tocilizumab in COVID‐19: an observational study. J Allergy Clin Immunol. 2020;147(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manson JJ, Crooks C, Naja M, et al. COVID‐19‐associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10):e594‐e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang G, Wu C, Zhang Q, et al. C‐reactive protein level may predict the risk of COVID‐19 aggravation. Open Forum Infect Dis. 2020;7(5):ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vargas‐Vargas M, Cortés‐Rojo C. Ferritin levels and COVID‐19. Rev Panam Salud Pública. 2020;44:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID‐19 patients. Int J Antimicrob Agents. 2020;56(2):106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gage JR, Fonarow G, Hamilton M, Widawski M, Martínez‐Maza O, Vredevoe DL. Beta blocker and angiotensin‐converting enzyme inhibitor therapy is associated with decreased Th1/Th2 cytokine ratios and inflammatory cytokine production in patients with chronic heart failure. Neuroimmunomodulation. 2004;11(3):173‐180. [DOI] [PubMed] [Google Scholar]
- 63. Bernstein KE, Khan Z, Giani JF, Cao D‐Y, Bernstein EA, Shen XZ. Angiotensin‐converting enzyme in innate and adaptive immunity. Nat Rev Nephrol. 2018;14(5):325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Horne JR, Vohl M‐C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS‐CoV illness severity. Am J Physiol‐Endocrinol Metab. 2020;318(5):E830‐E833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ni W, Yang X, Yang D, et al. Role of angiotensin‐converting enzyme 2 (ACE2) in COVID‐19. Critical Care. 2020;24(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yao Y, Cao J, Wang Q, et al. D‐dimer as a biomarker for disease severity and mortality in COVID‐19 patients: a case control study. J Intensive Care. 2020;8(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang S, Liu Y, Wang X, et al. SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. J Hematol Oncol. 2020;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stefanini GG, Chiarito M, Ferrante G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID‐19. Heart. 2020;106(19):1512‐1518. [DOI] [PubMed] [Google Scholar]
- 72. Tsolaki V, Zakynthinos GE. Are patients with COVID‐19 dying of or with cardiac injury? Am J Respir Crit Care Med. 2020;202(2):300‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Basso C, Leone O, Rizzo S, et al. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827‐3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gidding SS, Bacha F, Bjornstad P, et al. Cardiac biomarkers in youth with type 2 diabetes mellitus: results from the TODAY Study. J Pediatr. 2018;192:86‐92.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cavender MA, White WB, Jarolim P, et al. Serial measurement of high‐sensitivity troponin I and cardiovascular outcomes in patients with type 2 diabetes mellitus in the EXAMINE Trial (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care). Circulation. 2017;135(20):1911‐1921. [DOI] [PubMed] [Google Scholar]
- 76. Berezin AE, Berezin AA. Circulating cardiac biomarkers in diabetes mellitus: a new dawn for risk stratification—a narrative review. Diabetes Ther. 2020;11(6):1271‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15(2):117‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. von Lueder TG, Krum H. RAAS inhibitors and cardiovascular protection in large scale trials. Cardiovasc Drugs Ther. 2012;27(2):171‐179. [DOI] [PubMed] [Google Scholar]
- 79. Shi S, Qin M, Yang B. Coronavirus disease 2019 (COVID‐19) and cardiac injury—reply. JAMA Cardiol. 2020;5(10):751‐753. [DOI] [PubMed] [Google Scholar]
- 80. Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID‐19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ohkuma T, Jun M, Rodgers A, et al. Acute increases in serum creatinine after starting angiotensin‐converting enzyme inhibitor‐based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension. 2019;73(1):84‐91. [DOI] [PubMed] [Google Scholar]
- 82. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med. 2000;160(5):685‐693. [DOI] [PubMed] [Google Scholar]
- 83. Ryan MJ, Tuttle KR. Elevations in serum creatinine with RAAS blockade: why isnʼt it a sign of kidney injury? Curr Opin Nephrol Hypertens. 2008;17(5):443‐449. [DOI] [PubMed] [Google Scholar]
- 84. Tox U, Steffen H. Impact of inhibitors of the renin‐angiotensin‐aldosterone system on liver fibrosis and portal hypertension. Curr Med Chem. 2006;13(30):3649‐3661. [DOI] [PubMed] [Google Scholar]
- 85. Zhang H, Zhang X, Hou Z, Deng F. RhACE2 – playing an important role in inhibiting apoptosis induced by Ang II in HUVECs. Medicine. 2019;98(22):e15799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Skopas V, Papadopoulos D, Trakas N, et al. Lactate dehydrogenase isoenzymes in patients with acute exacerbation of chronic obstructive pulmonary disease: an exploratory cross‐sectional study. Respir Physiol Neurobiol. 2021;283:103562. [DOI] [PubMed] [Google Scholar]
- 87. Kalra A, Hawkins ES, Nowacki AS, et al. Angiotensin‐converting enzyme inhibitors versus angiotensin II receptor blockers. Circulation. 2020;13(10):e007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmieder RE. Mechanisms for the clinical benefits of angiotensin II receptor blockers. Am J Hypertens. 2005;18(5):720‐730. [DOI] [PubMed] [Google Scholar]
- 89. First randomised trial backs safety of common heart drugs in COVID‐19 patients. www.escardio.org. https://www.escardio.org/The-ESC/Press-Office/Press-releases/LOPES. Accessed January 23, 2021.
- 90. Lopes RD, Macedo AVS, de B Silva PGM, et al. Continuing versus suspending angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)–The BRACE CORONA Trial. Am Heart J. 2020;226:49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized clinical trial. JAMA. 2021;325(3):254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mancia G, Rea F, Ludergnani M, et al. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Abajo FJ, Rodríguez‐Martín S, Lerma V, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395(10238):1705‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


