Abstract
Several descriptive studies have reported that higher neutrophil count (NC) may be correlated with poor prognosis in patients with confirmed COVID‐19 infection. However, the findings from these studies are limited by methodology and data analysis. This study is a cohort study. We nonselectively and consecutively collected a total of 663 participants in a Chinese hospital from January 7 to February 28. Standardized and two‐piecewise Cox regression model were employed to evaluate the association between baseline neutrophil count (bNC), neutrophil count change rate (NCR), and death. bNC had a U‐shaped association with death. In the range of 0.1 to ≤1.49 × 109/L (hazard ratio [HR] = 0.19, 95% confidence interval [CI] = 0.05–0.66) and >3.55 × 109/L of bNC (HR = 2.82, 95% CI = 1.19–6.67), the trends on bNC with mortality were opposite. By recursive algorithm, the bNC at which the risk of the death was lower in the range of >1.49 to ≤3.55 × 109/L (HR = 13.64, 95% CI = 0.25–74.71). In addition, we find that NCRs (NCR1 and NCR2) are not associated with COVID‐19‐related deaths. Compared with NCR, bNC has the potential to be used for early risk stratification in patients with COVID‐19 infection. The relationship between bNC and mortality was U‐shaped. The safe range of bNC was 1.64–4.0 × 109/L. Identifying the correlation may be helpful for early risk stratification and medical decision‐making.
Keywords: baseline neutrophil count, change rate, COVID‐19‐infection, nonlinearity
Highlight
Identifying the safe rage of bNC may be helpful for early risk stratification and medical decision‐making for COVID‐19 patients.
1. INTRODUCTION
SARS‐CoV‐2 (henceforth COVID‐19) 1 has become a disaster affecting 192 countries. As of March 24, the number of confirmed cases and new cases/day worldwide were 289,123 and 19,225, respectively, and the mortality rate was 4.50% (13,011/289,123). (Data comes from the official Centers for Disease Control and Prevention [CDC] data of various countries, and is compiled by the Phoenix News Network.) More terrifyingly, there are at present no effective drugs and standardized guidelines for the treatment of COVID‐19 infection. 2 , 3
At present, the clinical research on COVID‐19 is mainly descriptive, 3 and the research on the risk factors of clinical occurrence, development, and adverse prognosis of COVID‐19 is still in the starting stage. 4 Although several studies have suggested that older, increased neutrophil counts (NCs), decreased lymphocyte counts, and thrombocytopenia may be related to the poor prognosis 5 , 6 , 7 , 8 , 9 , 10 , 11 ; there are more risk factors that still need to be explored as early as possible. It can help clinicians to evaluate risk stratification earlier, and develop a timely and individualized treatment strategy.
The data for this study comes from the First People's Hospital of Jiangxia District, Wuhan. We collected 1066 patients diagnosed with COVID‐19 infection. Through data mining, we used patient death as the outcome and find that the baseline neutrophil count (bNC) is nonlinear with respect to mortality, after being adjusted for other covariates.
2. PARTICIPANTS AND METHODS
2.1. Study design
We designed this study as a retrospective cohort study design. We set bNC, NC change rates as the target‐independent variable, and set COVID‐19‐related death (dichotomous variable: 1 = survival, 0 = non‐survival) as a dependent variable.
2.2. Study population
We nonselectively collected consecutive cases from the First People's Hospital of Jiangxia District Wuhan City, China. To mitigate the potential concern on privacy, the identity information of participants was encoded as the nontraceable codes in this study. Data collection of participants was performed using hospital electronic medical record system. The local ethics committee of the First People's Hospital of JiangXia District approved this study. Informed consent of involving participants was not required because of the retrospective design of the study, and the analysis used anonymous data.
Initially, 1066 participants participated in this study; 443 participants were subsequently excluded from this study, leaving 623 cases for the final data analysis (see Figure 1 for details). The start time and end time of the clinical data of collection for these involving participants was January 7 and February 28, respectively. All patients were followed up until discharge. Inclusion criteria included adults (>14 years) who suffered from COVID‐19 infection. The diagnostic criteria are based primarily on diagnosis and treatment of novel coronavirus pneumonia (seventh edition). 4 In the guide, the diagnostic criteria of COVID‐19 were based on the virus RNA detection, the clinical characteristics, chest imaging, and the ruling out of common pathogen. Exclusion criteria included (1) those patients who were still hospitalized before February 29; (2) those with missing neutrophil data; (3) those who died during admission; (4) those who had previous gastrointestinal surgery; (5) those who were diagnosed with severe type of COVID‐19 after admission; and (6) those with tumor duration.
Figure 1.
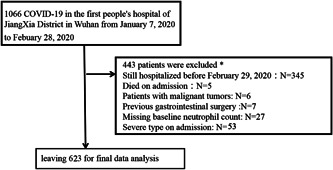
The flowchart of participant selection
2.3. Cured and discharge criteria
The body temperature returned to normal after 3 days, the respiratory symptoms significantly improved, the pulmonary imaging showed that the inflammation was obviously absorbed, and the respiratory pathogenic nucleic acid test was negative for two consecutive times (sampling interval was at least 1 day).
2.4. Variables
2.4.1. Neutrophil count
NCs measured at various time points were recorded as continuous variable. During the entire disease course, the different trend of NC between survivors and nonsurvivors were observed (Figure S1). Therefore, we used bNC, NC change rates as the target exposure variable. The NC change rate formula is as follows: NC change rate 1 (NCR1) = Absolute (NCsecond − NCbaseline)/NCbaseline; NC change rate 2 (NCR2) = Absolute (NCthird − NCbaseline)/NCbaseline. As bNC, NCR1, and NCR2 is skewed, we therefore converted them to log2 function. The detailed process to measure NC is described as follows: 2 ml of blood was drawn from the vein, and then put into an EDTA‐anticoagulation tube, and sent to the central laboratory of the hospital for analysis by the BC‐3000 autohematology analyzer (Mindray Medical International, Inc.).
2.4.2. COVID‐19‐related death
Our interesting outcome variable was COVID‐19‐related death (dichotomous variable: 1 = survival, 0 = nonsurvival).
2.4.3. Covariates
The covariates involved in this study were selected based on our clinical experience and studies of others examining risk factors for COVID‐19‐related death. 1 , 2 , 3 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Therefore, the following variables were used as covariates:
-
(1)
continuous variables: age (year), baseline lymphocyte count (×109/L), and baseline platelet count (×109/L).
-
(2)
categorical variables: sex (male/female), hypertension (yes/no), diabetes (yes/no), CAD history (yes/no), respiratory failure (yes/no), cardiac insufficiency (yes/no), cough (yes/no), and fever (yes/no).
2.5. Treatment protocol
Given that there are currently no standardized treatment guidelines for COVID‐19 pneumonia, clinical treatment is based on symptomatic and supportive treatment, including the use of glucocorticoids, antiviral drugs, oxygen inhalation, traditional Chinese medicine, and exacerbations mechanical ventilation therapy.
2.6. Statistical analysis
Baseline characteristics of participants are expressed as mean ± standard deviation (SD; Gaussian distribution) or median (range; skewed distribution) for continuous variables, and as percentages for categorical variables. χ 2 (categorical variables), one‐way analysis of variance (ANOVA) test (normal distribution), or Kruskal–Wallis H test (skewed distribution) were used to detect the differences among different bNC.
Our data analysis is based on the purpose of early risk stratification. Therefore, we used the baseline, the first two and the first three neutrophil change rates as exposure variables. Univariate and multivariate Cox proportional hazards regression model were employed to test the correlation between exposure variables and COVID‐19‐related death with three distinct models. Model 1 is the nonadjusted model with no covariates adjusted. Model 2 is the minimally adjusted model with only sociodemographic variables adjusted. Model 3 is the fully adjusted model with covariates presented in Table 1 (adjusted). As Cox proportional hazards regression model‐based methods are often suspected for their inability to deal with nonlinear models, on that account, nonlinearity between NC and COVID‐19‐related decease were addressed using Cox proportional hazards regression model with cubic spline functions and the smooth curve fitting (penalized spline method). If nonlinearity was detected, we first calculated the inflection point using recursive algorithm, and then constructed a two‐piecewise Cox proportional hazards regression model on both sides of the inflection point.
Table 1.
Baseline characteristics of the participants
| Quartile of log2 bNC | Q1 (<3.32 to ≤1.09) | Q2 (<1.09 to ≤1.68) | Q3 (<1.68 to ≤2.24) | Q4 (<2.32 to 5.03) | p value |
|---|---|---|---|---|---|
| N | 161 | 162 | 160 | 145 | |
| Age (year), mean (SD) | 45.53 (13.51) | 46.41 (13.89) | 47.71 (15.64) | 52.76 (14.91) | <.001 |
| Hospital stay (h), mean (SD) | 336.50 (140.28) | 336.74 (147.08) | 326.15 (146.66) | 326.70 (161.74) | .864 |
| Platelet count (×109/L), mean (SD) | 159.98 (56.38) | 176.78 (65.97) | 205.31 (74.85) | 238.06 (89.22) | <.001 |
| Lymphocyte count (×109/L) mean (SD) | 1.08 (0.44) | 1.10 (0.48) | 1.24 (0.65) | 1.21 (0.69) | .295 |
| Sex, n (%) | .065 | ||||
| Male | 60 (37.27%) | 76 (46.91%) | 83 (51.88%) | 64 (44.14%) | |
| Female | 101 (62.73%) | 86 (53.09%) | 77 (48.12%) | 81 (55.86%) | |
| Hypertension history, n (%) | .818 | ||||
| No | 132 (81.99%) | 139 (85.80%) | 133 (83.12%) | 122 (84.14%) | |
| Yes | 29 (18.01%) | 23 (14.20%) | 27 (16.88%) | 23 (15.86%) | |
| Diabetes history, n (%) | .213 | ||||
| No | 144 (89.44%) | 153 (94.44%) | 141 (88.12%) | 133 (91.72%) | |
| Yes | 17 (10.56%) | 9 (5.56%) | 19 (11.88%) | 12 (8.28%) | |
| Heart failure on admission, n (%) | .024 | ||||
| No | 159 (98.76%) | 158 (97.53%) | 153 (95.62%) | 134 (92.41%) | |
| Yes | 2 (1.24%) | 4 (2.47%) | 7 (4.38%) | 11 (7.59%) | |
| Coronary artery disease history, n (%) | .110 | ||||
| No | 158 (98.14%) | 160 (98.77%) | 158 (98.75%) | 138 (95.17%) | |
| Yes | 3 (1.86%) | 2 (1.23%) | 2 (1.25%) | 7 (4.83%) | |
| Fever on admission, n (%) | .260 | ||||
| No | 141 (87.58%) | 138 (85.19%) | 134 (83.75%) | 132 (91.03%) | |
| Yes | 20 (12.42%) | 24 (14.81%) | 26 (16.25%) | 13 (8.97%) | |
| Cough on admission, n (%) | .564 | ||||
| No | 146 (90.68%) | 147 (90.74%) | 150 (93.75%) | 136 (93.79%) | |
| Yes | 15 (9.32%) | 15 (9.26%) | 10 (6.25%) | 9 (6.21%) |
Abbreviation: bNC, baseline neutrophil count.
To ensure the robustness of our results, we performed a sensitivity analysis. We converted log2bNC into a categorical variable according to the quartile, and calculated the p for trend.
Modeling was performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). p < .05 (two‐sided) were considered statistically significant.
2.7. Missing data addressing
In our study, 27 cases of bNC were missing. To minimize selection bias caused by missing data, we performed a sensitivity analysis. The results showed that there was no statistical difference in the neutrophil missing and nonmissing groups (Table S1). For other covariates, because the percentage of missing data was 0%, no imputation was performed (Table S2).
3. RESULTS
3.1. Baseline characteristics of participants
The baseline characteristics of these included participants were indicated in Table 1. The mean age was 47.8 ± 14.7 years, 45.2% were male. The incidence of nonsurvival was 1.84% (12/653). No significant statistical difference in lymphocyte count, comorbidity (hypertension, diabetes, CAD history), and symptoms (fever and cough on admission) were detected across different groups of bNC (quartile; p > .05). When compared with Q1 group, older, higher platelet count, and lymphocyte count were observed in groups Q2, Q3, and Q4, while the opposite results were detected in covariates in terms of percent of respiratory failure and cardiac insufficiency (in Q4 group).
3.2. The results of multivariate analyses using Cox proportional hazards regression model
Model‐based hazard ratios, 95% CI and p values are listed in Table 2. In unadjusted model, an increase of 1 log2 of bNC was correlated with 121% increases in risk of COVID‐19‐caused mortality (2.21, 1.20–4.06). The results is statistically significant. In minimally adjusted model, when we only adjusted for demographic variables, each additional log2 of neutrophil at baseline increases by 80% (1.80, 0.95–3.42). In fully adjusted model, each additional log2 of bNC is matched by a 114% increases in nonsurvivor (2.14, 1.12–4.08). To verify the robustness of our findings, a sensitivity analysis was performed. We convert log2 neutrophil from a continuous variable to a categorical variable (according to quartile). The results show that the trend of the hazards ratio in different groups of log2‐bNC is inequidistant. The results obtained from the sensitivity analysis indicated that the clarification of nonlinearity between log2‐bNC on COVID‐19‐related death is necessary.
Table 2.
Results of univariate and multivariate analyses using Cox regression model
| Nonadjusted model HR, 95% CI, p value | Minimally adjusted model HR, 95% CI, p value | Fully adjusted model HR, 95% CI, p value | |
|---|---|---|---|
| Log2 bNC | 2.21 (1.20, 4.06) 0.0107 | 1.80 (0.95, 3.42) 0.0697 | 2.14 (1.12, 4.08) 0.0207 |
| Q1 | Ref | Ref | Ref |
| Q2 | 2.01 (0.18, 22.16) 0.5689 | 2.19 (0.19, 24.91) 0.5272 | 2.64 (0.23, 30.44) 0.4373 |
| Q3 | 3.04 (0.32, 29.26) 0.3354 | 2.30 (0.23, 22.59) 0.4741 | 3.44 (0.32, 36.78) 0.3072 |
| Q4 | 7.03 (0.85, 58.40) 0.0711 | 4.75 (0.57, 39.85) 0.1514 | 10.79 (1.10, 105.47) 0.0408 |
| p for trend | .0307 | .108 | .0234 |
| Log2 NCR1 | 0.77 (0.37, 1.60) 0.4773 | 0.77 (0.37, 1.60) 0.4808 | 0.70 (0.32, 1.53) 0.3764 |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.31 (0.22, 7.83) 0.7696 | 1.38 (0.23, 8.31) 0.7226 | 1.33 (0.20, 8.64) 0.7684 |
| Q3 | 1.75 (0.32, 9.54) 0.5202 | 2.69 (0.47, 15.31) 0.2648 | 2.66 (0.41, 17.23) 0.3055 |
| Q4 | 0.33 (0.03, 3.67) 0.3688 | 0.32 (0.03, 3.66) 0.3615 | 0.27 (0.02, 3.37) 0.3077 |
| p for trend | .4754 | .5363 | .4445 |
| Log2 NCR2 | 1.23 (0.93, 1.63) 0.1408 | 1.27 (0.95, 1.69) 0.1082 | 1.25 (0.90, 1.73) 0.1810 |
| Q1 | Ref | Ref | Ref |
| Q2 | 1.70 (0.15, 18.81) 0.6658 | 2.86 (0.25, 33.18) 0.4016 | 1.93 (0.14, 26.17) 0.6215 |
| Q3 | 1.25 (0.28, 5.58) 0.7716 | 1.19 (0.26, 5.37) 0.8236 | 1.30 (0.24, 7.08) 0.7623 |
| Q4 | 3.13 (0.35, 28.11) 0.3077 | 4.63 (0.48, 44.30) 0.1835 | 4.88 (0.46, 52.05) 0.1898 |
| p for trend | .3412 | .2583 | .2146 |
Note: Nonadjusted model: no covariates were adjusted; Nonadjusted model: no covariates were adjusted; Fully adjusted model: all covariates presented in Table 1 were adjusted for.
Abbreviations: bNC, baseline neutrophil count; CI, confidence interval; HR, hazard ratio; NCR, neutrophil count change rate.
For log2 NCR1 and log2 NCR2, no significant correlations were detected in unadjusted, minimally adjusted, and fully adjusted models. These findings demonstrated that in our data, there is no evidence that NCR1 and NCR2 are associated with COVID‐19‐related death.
3.3. The nonlinearity addressing by Cox proportional hazards regression model with cubic spline functions
Through the Cox proportional hazards regression model with cubic spline functions and smooth curve fitting, we observed that the correlation between log2‐bNC and death is nonlinear (Figure 2). Therefore, data were fit into a two‐piecewise Cox proportional hazards regression model (Table 3). By recursive algorithm, we first obtained the inflection point that was 0.58 and 1.83 of log2‐bNC (1.49 and 3.55 × 109/L of bNC, by inverse log2 logarithmic conversion). At the range of 0.1 to ≤1.49 × 109/L of bNC (by inverse log2 logarithmic conversion), the HR and 95% CI was 0.19, 0.05–0.66, respectively. At the range of >1.49 to ≤3.55 × 109/L of bNC, the HR and 95% CI was 13.64, 0.25–74.71, respectively. At the range of >3.55 × 109/L of bNC, the HR and 95% CI was 2.82, 1.19–6.67, respectively.
Figure 2.
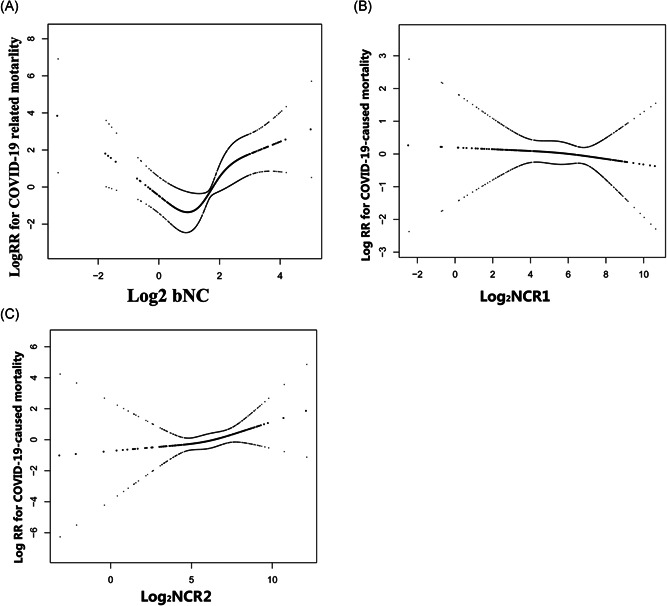
The nonlinearity addressing. (A) The U‐shaped association between bNC and death; (B and C) the association between NCR1 and NCR2 with death, respectively. bNC, baseline neutrophil count; NCR, neutrophil count change rate
Table 3.
Nonlinearity addressed through two‐piecewise linear model
| Model | HR, 95% CI, p value |
|---|---|
| Fitting model by standardized Cox regression | 2.14 (1.12, 4.08) 0.020 |
| Fitting model by two‐piecewise linear model | |
| Inflection point of log2 bNC | 0.58, 1.83 |
| ≤0.58 | 0.19 (0.05, 0.66) 0.0094 |
| >0.58 to ≤1.83 | 13.64 (0.25, 749.71) 0.2011 |
| >1.83 | 2.82 (1.19, 6.67) 0.0186 |
| Log likelihood ratio test | 0.030 |
Note: The adjustment strategy was the same as fully adjusted model.
Abbreviations: bNC, baseline neutrophil count; CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
In view of the previously reported descriptive studies that have mentioned the potential association between neutrophil elevation and poor prognosis, we therefore would like to clarify the following: (1) The trend of neutrophils count throughout the course of the disease. (2) Is there a link between neutrophils and death in patients with COVID‐19 infection? (3) Which time point (baseline? Short‐term change rate?) has more potential for future development of risk algorithms. In this retrospective observational cohort study, we examined the link of NC with mortality in Chinese patients diagnosed with COVID‐19 infection. We found that NCs in nonsurvivors show a time‐dependent gradual decline during hospitalization, while a stable trend of NCs in survivors are found. Compared with short‐term change rate of NC, bNC has more potential as a predictor for future prediction model development. In addition, the biggest gain of this study is the discovery of a U‐shaped relationship between bNC and death. Within the range of 1.6–4.0 × 109/L, patients have a relatively low risk of death. This helps physicians to assess the risk of poor prognosis in patients with COVID‐19 infection early.
Three previously published articles have addressed the association between bNC and clinical characteristics of COVID‐19 infection (study size: 59–452). Based on patients with COVID‐19, Qin et al. 13 reported that severe cases tend to have lower lymphocytes counts and higher leukocytes counts. Besides, Mo et al. 7 investigated 155 consecutive patients with confirmed COVID‐19 in Zhongnan Hospital of Wuhan University as the study population and obtained similar results. Li et al. 14 suggested that there was an increase in the proportion of neutrophils count in pregnant women (n = 41), children (n = 4), and nonpregnant women (n = 14) infected with COVID‐19. It is different from our findings. We attribute this difference to (1) the setting of the outcome variables. The outcome variable for this study was death; the outcome variable set by the abovementioned researchers was the type of disease (severe, non‐severe). (2) Most of the previous studies were cross‐sectional designs. Therefore, the choice of algorithm was logistic regression. However, this study was a cohort study. Therefore, considering the influence of time, we used Cox regression. In our data, Cox regression is more sensitive than logistic regression. (3) None of the previous studies have discussed nonlinear relationships, so nonlinearity cannot be found.
Although there is not much evidence, previous studies still tend to attribute the deterioration and death of patients with COVID‐19 infection to “cytokine storm” and immunologic abnormality, and these mechanisms also exist in respiratory syndrome (SARS), Middle East respiratory syndrome coronavirus (MERS‐CoV), and influenza A (H1N1). 15 , 16 , 17 , 18 A remarkable finding in our study was that both the increasing and depressed bNC was associated with fatal outcome of COVID‐19 patients. Neutrophil chemotaxis and transport play an important role in the host's immune defense process, but excessive infiltration of neutrophils can cause harmful inflammation. This excessive infiltration may have a profound interaction with the cytokine storm during virus invasion. 19 , 20 , 21 Therefore, an increase in bNC associated with an increased risk of death could be explained. The mechanism of COVID‐19 infection, leading to neutropenia has not yet been elucidated. However, excessive reduction of neutrophils often leads to subsequent immunodeficiency (similar to bone marrow suppression after chemotherapy), and increase the risk of coinfected with bacteria due to low immune function.
Some challenges may be raised. First, all patient data are collected after admission. It means that prehospital interventions (administration of antibiotics) can interfere with the accuracy of NCs. However, the effect of antibiotics on neutrophils is to reduce their concentration in peripheral blood, and our finding shows that the relationship between high neutrophils and COVID‐19‐associated death is positively correlated; that is, high neutrophils can increase the risk of death. Therefore, the effect of antibiotics on neutrophils is opposite to our results, but will make our results more strong. Second, giving patients antipyretics before admission can cause misclassification of fever. However, the results of the multiple Cox regression model indicated that fever can increase the risk of death in patients with COVID‐19 infection. Therefore, the bia direction of antipyretic was biased toward the reduction of mortality, which would lead to the result biased toward null. In addition, for patients with COVID‐19 infection, there is no evidence on the effect of antipyretics on NCs. Thus, even if patients were misclassified as having fever due to oral antipyretics, the impact of this misclassification on the association of bNC with COVID‐19‐related mortality is unknown. Lastly, changes in the patient's condition will cause neutrophils to change over time, and this time‐dependent variability will affect the results. However, by analyzing the time‐dependent trend of neutrophils at three time points, the results showed that there was no statistical difference between the neutrophils of the third time and those of the second time (Figure S2). Therefore, our setting of the neutrophil change rate does not need to consider the effect of the significant difference between the second and third neutrophils on the change rate.
Our study have some strengths of note. (1) Our study has a large sample size, whereas most prior studies were limited to a small number. (2) Compared with the previous research, research on the nonlinearity of addressing is a significant improvement. We obtain the safe range of bNC in COVID‐19 patients. This greatly improves the clinical value of this study. (3) This study is an observational study and therefore susceptible to potential confounding. We used strict statistical adjustment to minimize residual confounders. (4) In this study, we tested the robustness of the results through a series of sensitivity analyses (target‐independent variable transformation, log‐likelihood ratio test, etc.) to ensure the reliability of the results.
Our research has the following limitations. (1) The participants involved in this study are all Chinese. Therefore, our findings should be interpreted with caution because it is ethnically restrictive. (2) We excluded patients with cancer and those who had previously undergone gastrointestinal surgery, so the results did not apply to these patients. (3) We excluded patients diagnosed with severe type on admission. It well known that the mortality rate of severely affected patients is extremely high. Therefore, excluding these patients would have biased our results to the null and made our results stronger. (4) Detection of neutrophils is through an automatic blood cell analyzer. It cannot be ruled out that specimens cause blood cell fragmentation and deviation of neutrophil results during transportation and inspection. However, it does not cause measurement bias. (5) As in all observational studies, even though known potential confounders factors were controlled for, there might have been still uncontrolled confounders. (6) This study did not adjust treatment‐related indicators. However, such nonadjustment of treatment‐related indicators would have biased our results to null. (7) Data collection and analysis in the present were performed at the early stage of the COVID‐19 outbreak. Therefore, some variables that have been proven to be related to the prognosis of COVID‐19 in subsequent studies have not been included.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Kun Wang and Chengyun Liu conceived and designed the study. Kun Wang, Peiyuan Zuo, Chi Chen, and Xin‐Lin Chen analyzed the data. Chi Chen wrote the first draft of the manuscript. Kun Wang, Yuwei Liu, Meng Zhang, Xiaofang Zhao, Songpu Xie, and Hao Zhang recruited patients, gathered data, and participated in manuscript revision. Chengyun Liu provided study oversight and participated in manuscript revision. All authors had access to study data and approved the decision to submit the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26794.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grants: 81671386 and 81974222) and the Foundation of Guiyang Municipal Science and Technology Bureau ([2020]No.6). We thank the patients and their families who agreed to participate in this important study. We thank Juan Feng and Haibin Han (Department of Information Center, The First People's Hospital of Jiangxia District, Wuhan City and Union Jiangnan Hospital, Huazhong University of Science and Technology, Wuhan, China) for their assistance in establishing the clinical database.
Fu W, Chen C, Chen X‐L, et al. A U‐shaped association between baseline neutrophil count and COVID‐19‐related mortality: A retrospective cohort study. J Med Virol. 2021;93:4265‐4272. 10.1002/jmv.26794
Chi Chen and Xin‐Lin Chen contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lake MA. What we know so far: COVID‐19 current clinical knowledge and research. Clin Med (Lond). 2020;20(2):124‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. General Office of the National Health Commission of the People's Republic of China, Office of the State Administration of Traditional Chinese Medicine . Diagnostic and therapeutic protocol for novel coronavirus pneumonia. China National Med. 2020;15(6):801‐805. [Google Scholar]
- 5. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in Northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qu R, Ling Y, Zhang Y, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92:1533‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;Mar 16:ciaa270. [Google Scholar]
- 8. Wu WS, Li YG, Wei ZF, et al. Investigation and analysis on characteristics of a cluster of COVID‐19 associated with exposure in a department store in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(4):489‐493. [DOI] [PubMed] [Google Scholar]
- 9. Dong XC, Li JM, Bai JY, et al. Epidemiological characteristics of confirmed COVID‐19 cases in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):638‐642. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Su X, Chen W, et al. Epidemiological investigation on a cluster epidemic of COVID‐19 in a collective workplace in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):649‐653. [DOI] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramadass M, Johnson JL, Marki A, et al. The trafficking protein JFC1 regulates Rac1‐GTP localization at the uropod controlling neutrophil chemotaxis and in vivo migration. J Leukoc Biol. 2019;105(6):1209‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug‐of‐war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9(1):558‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—an update on the status. Mil Med Res. 2020;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tetro JA. Is COVID‐19 receiving ADE from other coronaviruses?. Microbes Infect. 2020;22(2):72‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol. 2017;39(5):541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janice OH, Ken‐En GS, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg Microbes Infect. 2012;1(9):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enjuanes L, Almazán F, Sola I, Zuñiga S. Biochemical aspects of coronavirus replication and virus–host interaction. Annu Rev Microbiol. 2006;60(1):211‐230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


